Introduction
When embracing a novel surgical innovation, it becomes imperative to assess its long-term outcomes and safety using comprehensive and reliable data. Clinicians must prioritize the study of excellent peer-reviewed articles rather than being influenced by marketing materials or non-peer-reviewed sources. Proceeding with caution when adopting new technologies is wise, as past refractive procedures have been discontinued due to their inadequate effectiveness or the emergence of delayed complications (as shown in Table 1).
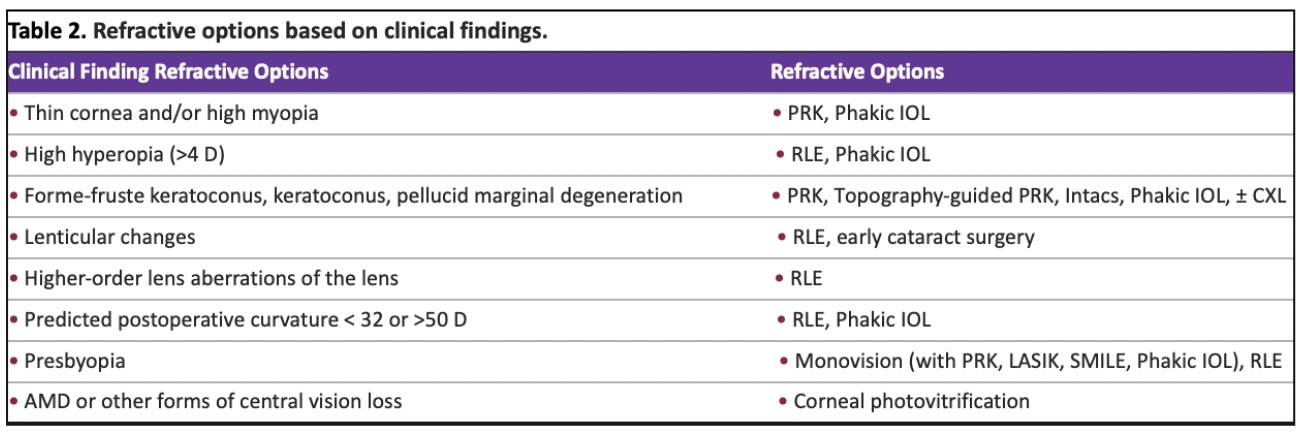
Patients generally have high expectations today, as they desire post-operative uncorrected visual acuity (UCVA) to be equal or better than their preoperative best spectacle corrected visual acuity (BCVA). The role of the clinician is to thoroughly evaluate the patient’s ocular health and determine their suitability for refractive procedures. Preoperative assessments provide valuable insights to guide the surgeon in recommending specific refractive options (Table 2). By distinguishing between higher order aberrations originating from the cornea and the lens, advanced wavefront units enable the measurement of total higher order aberrations of the eye. In cases where significant higher-order aberrations are found in the lens, a refractive lens exchange becomes the preferred treatment option to enhance overall visual quality.
Laser Vision Correction: Laser-assisted in situ keratomileusis (LASIK) and photorefractive keratectomy (PRK)
Over 35 million LASIK and PRK procedures have been performed worldwide, demonstrating significant advancements in outcomes and safety.1 The past three decades have witnessed remarkable progress in excimer laser technology, including improved nomograms, smoother ablations through flying spot lasers, more precise trackers, larger optical zones, aspheric curves, and customized treatments that address not only refractive errors but also other optical aberrations of the eye.
PRK has proven to yield excellent outcomes comparable to LASIK. While some surgeons prefer PRK due to its lower risk of corneal ectasia, most initially recommend LASIK for its faster post-operative healing. Surgeons typically opt for PRK when the cornea is thin, mildly irregular, or exhibits signs of epithelial basement membrane dystrophy. PRK may also be favored when flap creation is complicated by a narrow fissure or when there is a higher risk of flap subluxation due to occupational or sporting factors. PRK improves both day and night vision quality while preserving corneal clarity. This is achieved through the use of larger optical zones, smoother ablations using flying spot lasers, adjunctive application of mitomycin C to reduce the risk of corneal haze, and custom treatments such as topography- and wavefront-guided ablations, as well as wavefront-optimized approaches. Custom ablation with PRK achieves refraction outcomes comparable to small-incision lenticule extraction (SMILE), but with fewer induced higher-order aberrations.1 Following PRK, patients experience a relatively comfortable recovery with the application of sterile ice on the corneal surface, bandage soft contact lenses, and nonsteroidal drops.
LASIK stands out as one of the most commonly performed and successful surgical procedures.2,3 With thorough preoperative screening, visual outcomes are excellent and complications are rare. In North America, femtosecond lasers have largely replaced blade microkeratomes for creating the corneal flap. Advances in femtosecond technology have resulted in predictable flap thickness and the ability to customize the diameter, location, hinge, and edge profile of the LASIK flap. In the unlikely event of suction loss during femtosecond laser application, the suction ring can be reapplied, and the procedure can proceed.
Conversely, if suction is lost with a mechanical microkeratome, the procedure is halted, and the patient must return for PRK several months later. In a comprehensive clinical review of LASIK (97 papers; 67,893 eyes) conducted between 2008 and 2015, the results showed remarkable success rates.4 Of the eyes included in the review, 90.8% achieved a distance uncorrected visual acuity (UCVA) of 20/20, and 99.5% achieved a UCVA of 20/40 or better. The spherical equivalent refraction was within ±0.50 D of the target in 90.9% of eyes and within ±1.00 D of the target in 98.6% of eyes. These outcomes surpassed earlier reports, reflecting advancements in laser hardware and software, surgical techniques, and improved patient selection.
The review also revealed that only 0.61% of eyes experienced a loss of two or more lines of corrected distance visual acuity (CDVA), which was significantly lower than the number of eyes that gained two or more lines of CDVA (1.45%). Advanced treatment options, such as topography- and wavefront-guided or wavefront- optimized approaches, resulted in nearly a full line improvement in uncorrected distance visual acuity(UDVA)compared to conventional treatment.5,6 The majority of treatments reviewed focused on myopia and myopic astigmatism, while hyperopic treatments represented only 3 %of the cases.4 However, hyperopic treatments had a higher incidence (2.13%) of a ≥2-line CDVA loss compared to myopic treatments (0.95%). This may be attributed to the increased sensitivity of centration in hyperopic treatments, which should ideally align with the line of sight rather than the center of the pupil. Hyperopic treatments also carried a greater risk of regression compared to myopic treatments.
Corneal ectasia is recognized as the most significant long-term complication of LASIK, with an incidence of approximately 0.03%.7 However, advancements in preoperative detection using elevation tomography to identify elevation abnormalities on the anterior and posterior corneal surfaces have helped reduce this risk.8 Other factors contributing to improved LASIK outcomes include avoiding surgery on thin corneas or those with high myopia, creating thinner corneal flaps, and leaving a thicker residual bed under the flap.9,10 In cases of corneal ectasia, the preferred treatment is corneal crosslinking, potentially combined with topography-guided PRK or the use of intracorneal rings to reduce irregular astigmatism.11 Early detection and treatment of ectasia can minimize corneal irregularities and improve visual acuity.
Continued research in tracking devices, torsional alignment, optimal centration of ablations, a deeper understanding of corneal biomechanical properties, and the exploration of medications or adjunctive procedures to modulate wound healing will further enhance outcomes and ensure patient safety across all laser vision correction procedures.
Intrastromal Lenticule Extraction
Intrastromal Lenticule Extraction is an innovative technique for intrastromal keratomileusis that involves the use of a femtosecond laser to create two cuts within the cornea and one small superficial cut.12,13 The procedure was developed by Zeiss and termed SMILE, which stands for Small Incision Lenticule Extraction.
The Intrastromal Lenticule Extraction procedure generates a lenticule of defined shape and thickness, which is then extracted mechanically through a small corneal incision with a diameter of 2-3 mm. The procedure presents itself as an alternative refractive procedure specifically designed for correcting myopia and myopic astigmatism. Recent studies have confirmed its effectiveness and safety, establishing it as a viable option in vision correction.13 For a detailed comparison between LASIK, PRK, and SMILE, please refer to Table 3.
Currently, the SMILE procedure is primarily reserved for treating myopia and myopic astigmatism. When enhancement procedures are necessary, they typically involve PRK.14 However, recent evidence suggests that LASIK could also be effective in correcting residual refractive errors following SMILE. It’s important to note that there are certain limitations associated with first-generation SMILE in comparison to LASIK. These limitations include challenges in performing low myopic corrections (below 3 diopters) due to the thin and delicate nature of the lenticule, ineffectiveness in addressing hyperopia or hyperopic astigmatism, inability to perform topography- or wavefront-guided treatments, less precise cuts with femtosecond lasers compared to excimer lasers, absence of optical centration adjustment when the suction device is applied to the eye, lack of cyclotorsion compensation, slower recovery of uncorrected visual acuity (UCVA), and less significant improvement in best UCVA when compared to customized LASIK treatments.15–17
Clinical studies comparing SMILE to custom LASIK, using either wavefront-guided18 or topography-guided techniques,19–21 have consistently demonstrated superior outcomes with LASIK. This superiority primarily relates to reduced induced high-order aberrations, leading to an improved quality of vision. Despite these limitations, advancements in laser technology and software design are expected to enhance the outcomes of the Intrastromal Extraction procedure.
A meta-analysis compared SMILE (291 eyes) to femtosecond LASIK (FS-LASIK; 277 eyes) for correcting myopia in patients with dry eye.22 The study concluded that both SMILE and LASIK temporarily induce dry eye, and while SMILE exhibits certain early postoperative advantages, it doesn’t demonstrate long-term superiority over FS-LASIK in terms of tear characteristics, tear quantity, or subjective symptoms. However, earlier meta-analyses suggested a lower risk of postoperative dry eye with SMILE.23,24
Both SMILE and LASIK procedures can lead to decreased corneal sensation due to the creation of the flap and subsequent ablation. Research findings on the risk of dry eye as a result of corneal neuralgia are somewhat mixed.24,25 While some randomized controlled studies indicate no heightened risk, other reports suggest an association.26,27 A particular meta-analysis indicated that corneal sensitivity recovery in the SMILE group is faster than in the FS-LASIK group during the initial three months post-surgery, but recovery levels are similar at the six-month mark.28
Maintaining corneal biomechanical properties is crucial for stable refractive correction and preventing corneal ectasia. Both SMILE and LASIK procedures reduce corneal tensile strength by removing corneal tissue, with the extent of reduction correlating to the level of myopic ablation.29 SMILE seems to result in a greater reduction in tensile strength for lower myopic corrections due to increased tissue removal, but similar reductions occur for higher corrections.29 Theoretical models, mathematical analysis, and experiments on cadaver corneas suggest that SMILE might better preserve corneal biomechanical properties compared to LASIK.30,31 Given that iatrogenic ectasia is a rare complication, it is advisable for the indications and exclusion criteria for SMILE to align with those of LASIK.
Recent advances have led to the introduction of the new SILK Procedure (Smooth Incision Lenticule Keratomileusis) from Johnson & Johnson. This technology uses contiguous spots of ultralow laser pulse energy and has a number of theoretical advantages to be confirmed in the peer-review literature (Table 4). These advantages include a smoother cut, easier lenticule removal, improved predictability of depth of cut, less dry eye secondary to a different shape of extracted tissue, and better preservation of biomechanical strength of the cornea with faster nerve regeneration due to less corneal nerves and fibrils being cut. Other companies are also set to introduce lasers for Intrastromal Lenticular Extraction procedures, bearing names such as CLEAR (Ziemer) and SMART SIGHT (Schwind).
Phakic IOL
Phakic IOL insertion is the preferred procedure for treating high levels of myopia and astigmatism, particularly in nonpresbyopic individuals with clear natural lenses. Unlike laser vision correction, this procedure is reversible as it does not involve tissue removal and does not induce dry eye symptoms. Phakic IOLs can be placed in two locations: the anterior chamber, such as iris-claw intraocular implants, or the posterior chamber. It is worth noting that anterior-chamber iris-supported phakic IOLs have been associated with a higher risk of corneal endothelial cell loss compared to posterior-chamber phakic IOLs.30
Implantable contact lenses (ICLs) have demonstrated excellent outcomes and high patient satisfaction rates.32–34 These lenses are made of a collamer substance that provides ultraviolet (UV) protection. The latest innovation in this field is the EVO Visian ICL (Figure 1) by Staar Surgical, which features a microscopic hole in the optic of the lens to prevent pupillary block glaucoma. This advancement eliminates the need for a preoperative yttrium aluminum garnet (YAG) laser iridotomy, which previously required the creation of two openings. The presence of the hole also reduces the risk of developing an anterior cortical cataract by enhancing fluid flow in the anterior chamber.35 Long-term data on EVO ICL designs have demonstrated a low incidence of cataracts. The EVO Visian ICL is designed for correcting myopia of up to 20 D and astigmatism of up to 6 D. A non-EVO ICL is available for correcting hyperopia; however, many hyperopic individuals may not be suitable candidates due to narrow anterior chambers. One notable indication for the use of the hyperopic ICL is in patients who have undergone radial keratotomy, as they have myopic eyes with deeper anterior chambers.
Clinical trials are currently underway for an extended depth-of-focus (EDOF) ICL. This type of lens holds potential for meeting the vision needs of young presbyopic patients who desire a full range of vision without lenticular changes or significant intraocular higher-order aberrations.
Refractive Lens Exchange (RLE)
Refractive Lens Exchange (RLE) has gained popularity due to advancements in micro- incisional phacoemulsification, precise optical biometry, improved intraocular lens (IOL) formulas, and innovative IOL designs.36,37 It is the preferred choice for patients with early lenticular changes, significant higher-order lens aberrations, high hyperopia, high myopia in eyes unsuitable for a phakic implant due to a shallow anterior chamber or low endothelial cell count, and presbyopes seeking improvement in distance, intermediate, and near vision. The advent of multifocal implants and the technique of monovision has allowed presbyopic patients to achieve a full range of vision. Monofocal implants are also available to reduce spherical aberration and correct astigmatism. A broader range of IOL powers, including high negative powers for high myopes and high positive powers for high hyperopes, further enhances the options for RLE. One notable advantage of RLE is the elimination of the need for future cataract surgery.
The correction of presbyopia is a significant goal in ophthalmology, and RLE with a multifocal or extended-depth of focus IOL is commonly used for this purpose. However, it is crucial to discuss the risks and benefits of surgery with the patient. Multifocal IOLs may lead to symptoms such as glare, halos, and reduced contrast sensitivity. With time, these optical aberrations typically improve due to the brain mechanism of neuroadaptation.38,39
Similar to cataract surgery, RLE carries the potential for vision- threatening complications like chronic cystoid macular edema, endophthalmitis, or retinal detachment. In younger patients with high myopia and long axial lengths who have not undergone complete posterior vitreous detachment, retinal detachment is a primary concern.40 The reported outcomes of RLE are comparable in young and older presbyopes, encompassing visual and refractive outcomes, complication rates, and patient satisfaction.41
Approximately 75% of patients undergoing lens-based surgery have astigmatism equal to or greater than 0.5 D.42 A residual astigmatism of 0.75 D or higher can negatively impact visual function and patient satisfaction. Toric implants are more effective than spherical implants and limbal relaxing incisions in reducing astigmatism.43 IOL formulas that incorporate empirical measurements of the posterior corneal curvature to determine total corneal astigmatism, such as the Barrett Toric formula, have yielded more accurate postoperative results.43 Advances in posterior corneal curvature measurements, facilitated by devices like the Cassini and IOLMaster 700, are enhancing the accuracy of these measurements.44 However, the accuracy of these devices compared to empirical measurements remains a subject of conflicting reports.
Patients who have previously undergone laser vision correction and are now experiencing presbyopia often seek improvements in their intermediate and near vision while fine-tuning their distance vision. These individuals commonly prefer multifocal or extended-depth of focus implants to achieve a broader range of vision. However, determining the most suitable approach for each patient requires a comprehensive evaluation of their visual needs and expectations.
Determining the optimal power of an intraocular lens (IOL) can be challenging, but advancements have been made with the utilization of the Barrett True-K formula, particularly for patients who have undergone LASIK or PRK in the past. In the future, the preferred technique for accurately measuring total corneal astigmatism and axis will likely involve evaluating both the anterior and posterior corneal surfaces.
Surgeons have a wide range of choices when it comes to selecting monofocal, toric, extended-depth of focus, segmented bifocal, and multifocal IOLs to cater to the specific needs of their patients. Procedures that involve replacing the natural lens, such as refractive lens exchange, continue to be widely accepted as an effective method for correcting presbyopia.
The femtosecond laser (Figure 2) can be used for RLE surgery to create a perfectly round capsulotomy, fragmentation of the nucleus, and limbal relaxing incisions at an exact depth, length, and angle to correct low levels of astigmatism. Although outcomes have been reported to be similar to manual surgery, there is evidence of better quality of vision in eyes with the femtosecond laser.45 This is secondary to the creation of a perfectly round capsulotomy, which holds the implant in position, decreasing tilt and decentration (Figure 3).
Extended Depth of Focus Implants
EDOF (Extended Depth of Focus) IOLs are known to produce fewer issues with glare, halos, and loss of contrast compared to multifocal IOLs.46 They offer excellent uncorrected distance and intermediate vision, although near vision may be better with multifocal implants.Surgeons who use EDOF IOLs often employ a micro-monovision technique, where the EDOF lens is placed in the dominant eye and the other eye is left slightly myopic to enhance reading ability. The two most popular implants in this category are the Vivity implant (Alcon) and the Eyhance (Johnson and Johnson). The latter implant is technically a mononfocal plus implant but has shown very positive outcomes in terms of a broader range of vision.
Segmented bifocal IOLs
Segmented bifocal IOLs, such as the SBL-3 (Lenstec), offer two focus zones for either distance or reading.47,48 Similar to multifocal implants, patients may experience glare, especially at night or if there is a residual refractive error. Refraction measurements can be more challenging with segmented bifocal lenses, as patients will have refractive errors through both the distance optic and the segmented bifocal region.
Accommodating IOLs
Accommodative IOLs have had limited success in providing satisfactory near vision. Examples of such lenses include the Crystalens AO (Bausch + Lomb), Tetraflex (Lenstec), and the dual-optic IOL like the Synchrony (AMO). Future accommodative IOLs currently in design or clinical study aim to offer patients the potential of a full range of vision without issues like glare, halos, or compromised visual quality.
FluidVision IOL
The FluidVision IOL (Alcon, formerly PowerVision), which is undergoing clinical trials, allows the quantity of fluid within the optic to be adjusted, thereby changing its accommodative power.49 When the ciliary muscle relaxes, fluid is displaced centrally into the lens, expanding the central membrane and facilitating near vision. Conversely, when the ciliary muscle relaxes, fluid returns to the periphery, creating distance vision. The lens has shown promising results outside of North America, with reports of accommodative amplitude of up to 2.2D observed over a three-year follow-up period.49
Sapphire IOL
The Sapphire IOL (ELENZA) incorporates cutting-edge nanotechnology and advanced electronics, enabling it to dynamically adapt its focus based on changes in the pupil size (Figure 5).49 Through the implementation of artificial intelligence, it can effectively distinguish between light stimulation and accommodation by analyzing the speed and magnitude of pupillary responses, resulting in near vision for the patient.
The implant utilizes pupillary responses to initiate changes in the liquid crystal within the lens, thereby modifying its refraction. By precisely assessing the speed and amplitude of the pupil’s response, the lens can discern between light and accommodative stimuli, optimizing visual outcomes.
To ensure convenient usage, the lens is equipped with a power-cell that requires recharging approximately every three-to-four days. In case of power depletion, it enters a hibernation mode and activates a fail- safe mechanism, temporarily converting it to monofocal status until recharged.
Furthermore, the ELENZA Sapphire IOL offers additional flexibility by allowing the physician to remotely adjust the sensitivity and magnitude of the switching point for the add power in the lens, tailored to the specific needs of the patient. This feature ensures personalized and adaptable vision correction for an enhanced post-operative experience.
Lumina lens
The Lumina lens (AkkoLens), is a sulcus-implanted accommodative IOL, which consists of two optical elements that move in response to ciliary muscle contraction (Figure 6).
These elements, stacked on top of each other, produce accommodation.49 The anterior element provides a fixed optical power of 5 D, while the posterior element offers 10-25 D. The lens can be inserted through a 2.8-3.0 mm incision. A 12-month clinical trial has shown significantly enhanced near vision compared to a monofocal IOL, while maintaining similar contrast sensitivity.49
JelliSee
The latest addition to presbyopic IOLs currently in development, is the JelliSee (JelliSee Ophthalmics) accommodating IOL (Figure 7). It is a monofocal lens, nearing the completion of its preclinical phase. The design allows the implant to adapt to the natural forces of the ciliary muscle, much like the technology employed in the FluidVision, as the eye shifts its focus between near and distant targets.50 This adaptability is achieved through the lens flexibility, which responds to the muscle’s actions. As the ciliary muscle relaxes, the JelliSee lens flattens and increases in diameter, responding to the force exerted by the zonules on the lens capsule. This enables changes in focus from near to far vision.
Promising results from initial bench studies conducted by the company indicate that the JelliSee lens can achieve 6 diopters or more of accommodation with a mere 0.2 mm or less of diameter change. Moreover, the lens provides smooth and immediate transitions across all ranges of vision (near, distance, and intermediate), with minimal dysphotopsias and negligible impact on contrast sensitivity.
One of the advantages of the JelliSee IOL is its independence from retained capsular elasticity. This means that even in the presence of capsular fibrosis, the lens’s functionality remains unaffected, ensuring consistent and reliable performance for patients in need of presbyopic correction.
Small-aperture IOLs
Small-aperture IOLs can extend the depth of focus, particularly benefiting patients with significant higher-order aberrations resulting from conditions like post-RK, keratoconus, or irregular corneal surfaces. The IC-8 IOL (AcuFocus) is a monofocal IOL designed with a pinhole aperture, similar to the KAMRA corneal inlay, to enhance depth of focus (Figure 8).51–53 It features a central aperture of 1.36 mm surrounded by a 3.23 mm opaque area and is inserted into the capsular bag. Clinical results have demonstrated good distance, intermediate, and near vision, especially when targeting up to -0.75 D of myopia.
Light-adjustable Lens
The light-adjustable lens (LAL) is an innovative lens made of photosensitive silicone material and can be fine-tuned postoperatively using UV light.54,55 After the standard IOL measurements and surgical procedure, if the power of the IOL is not optimal, adjustments can be made to correct the sphere from -2.00 to +2.00 D or astigmatism up to 2 D, thereby refining the patient’s uncorrected vision. Additionally, the implant has high negative spherical aberration, which increases the depth of focus enhancing near vision. Patients are required to wear specialized UV protecting sunglasses postoperatively during all waking hours both indoors and outdoors to protect the implants from UV light. After a number of weeks, a few laser treatments are performed to correct any residual prescription and lock in the effects. While there are theoretically many possible indications including routine cases, the most prevalent ones involve patients who require more intricate implant calculations, particularly in cases following laser vision correction or radial keratotomy.
One significant drawback of LALs involves the considerable time investment they demand. Although the actual treatments are relatively swift, taking just a minute or two, patients must undergo numerous post- operative appointments and specialized light therapies following the surgery. Additionally, as patients require refractive and dilation procedures, each visit might also consume a substantial amount of time.
The process of laser adjustment can prove to be challenging when dealing with inadequate pupil dilation. Given that proper light adjustments necessitate a comprehensive view of the lens optic, the pupil needs to be dilated to a minimum diameter of 5.3 mm. Failing to achieve this dilation could result in a section of the lens periphery being obscured by the iris, which could contain untreated macromers.
Corrections of refractive errors in pseudophakes
Improving a patient’s vision following lens implant surgery can be achieved through various methods such as IOL exchange, laser vision correction, or the use of a secondary IOL in the sulcus.56 Rayner Sulcoflex IOLs are piggyback lenses that can be custom-made to correct nearly any refractive error, including presbyopia (Figure 9).57 These lenses feature a 6.5 mm optic and a 14 mm length, and they are available in monofocal, toric, or trifocal designs. Pseudophakic patients that had a monofocal or trifocal implant inserted in the capsular bag, can now have the full range of vision allowing distance, intermediate, and near vision with the insertion of a piggyback.
Corneal Inlays
Corneal inlays have encountered several challenges in the past. Early inlays were associated with issues such as corneal opacification, vascularization, keratolysis, and decentration.58,59 To ensure long-term success, inlays need to be thin, have a small diameter, provide adequate nutritional and fluid permeability, and be inserted relatively deep into the cornea. The most commonly used inlays have been the KAMRA and Raindrop inlay. The KAMRA is a small aperture inlay that enhances the depth of focus and is inserted deep into the cornea. Insertion into a corneal pocket has shown better outcomes compared to placement under a LASIK flap. The KAMRA inlay is made of polyvinyl material, with a diameter of 3.8 mm, a thickness of 5 µm, and a 1.6-mm central opening. The Raindrop inlay, is made of acrylic material, measures 2 mm in diameter and 25 µm in thickness, is inserted deep in the cornea to induce central steepening to enhance reading ability.58,59
Unfortunately, the clinical results associated with corneal inlays have shown significant variability, leading many surgeons to discontinue the procedure due to early or late complications. The most common complication has been corneal haze, which often necessitated the removal of the inlay.
Clinical trials are currently being done using an allogenic corneal inlay (Figure 10). This has the potential to avoid the complications from synthetic inlays of corneal haze and overlying surface issues because of permeability issues.60,61 If clinical trials are successful, this corneal inlay may play an important role in the correction of presbyopia.
Early FDA clinical trial results of the TransForm (Allotex) corneal inlay have shown great potential in terms of safety, efficacy, and biocompatibility, as all pilot study patients experienced improved uncorrected near vision.60 Although four out of 12 patients experienced a slight decrease in uncorrected distance visual acuity and a mild myopic shift in refraction, their best-corrected distance visual acuity remained unchanged from preoperative levels. A published clinical trial in Turkey involved 50 eyes of 25 patients followed for 3 years showed good distance, intermediate, and near vision.62
To optimize its use, the inlay is placed in the nondominant eye for both near and distance vision. The inlay is made from eye bank corneal tissue that is not suitable for fresh transplantation. The process involves punching the donor tissue with a trephine to obtain buttons of the desired diameter, which are then sliced along a lamellar plane to create multiple tissue blanks. These blanks are precisely shaped using an excimer laser, decellularized, and stored in recombinant albumin solution. To ensure sterility, they are sterilized using electron beam radiation. Notably, a single donor cornea can yield a large number of inlays. These presbyopic inlays have a diameter of 3 mm, a thickness of 20 µm, and a target refractive add power of 2.5 D. They also boast a shelf life of up to 24 months at room temperature.
Topography-guided PRK and Corneal Crosslinking for Keratoconus, Pellucid Macular Degeneration, and Ectasia
Topography-guided PRK (TG-PRK) combined with Corneal Crosslinking (CXL) has been shown to improve best-corrected acuity in patients with irregular astigmatism from keratoconus, pellucid marginal degeneration, and ectasia.63,64 A series of topographic maps are performed and this data is used to drive the excimer laser to flatten areas of the cornea that are steep, and steepen areas that are flat (Figure 11). The best candidates for this technology have clear corneas without scarring, corneas greater than 430 microns, and a dioptric difference between the steepest point of the cornea and flattest point of less than 10 diopters. The technology has the potential to allow patients to see better with glasses or soft contact lenses. Following TG-PRK, CXL is performed to stiffen the corneal collagen fibers and prevent progressive disease.
Corneal Laser for Macular Degeneration
Corneal photovitrification (CPV) is a novel corneal laser procedure for improving vision in patients with age- related macular degeneration (AMD) and other conditions with central visual loss. This is a corneal refractive procedure that redirects light to preferred retinal locations in the macula with functioning photoreceptors (Figure 12). A 2017 case series demonstrated that a single treatment of CPV without visual training improved binocular and monocular near and distance vision effectively, safely, and comfortably from 1 to 12 months after CPV.65 The treatment did not cause peripheral field restriction, diplopia, or other adverse events in patients with low vision from atrophic or neovascular AMD. Canadian clinical experience with the first approved CPV device in North America also demonstrated improved visual function, enhanced quality of life, and excellent safety in both dry and wet AMD patients with no epithelial defects or other complications.66–68
Patient selection and testing for CPV are evolving, but this procedure offers the potential to help patients not only with AMD but also those with low vision from Best disease, Stargardt disease, macular holes and other retinal disorders with central loss of vision.
Summary
In summary, recent advancements in refractive surgery have revolutionized the field, offering patients greater independence from glasses and contact lenses with improved accuracy and safety. Innovations in laser vision correction, phakic implants, corneal inlays, TG-PRK, and RLE have significantly contributed to better outcomes. Furthermore, groundbreaking refractive techniques are enabling individuals with central vision loss to regain some functional vision by redirecting light from the cornea to functional photoreceptors.
Refractive surgery has gained widespread acceptance worldwide as an alternative to traditional visual aids, and efforts are underway to make these procedures accessible in underdeveloped regions as a primary vision care solution. With the rapid growth of knowledge, technology, and surgical expertise, refractive surgery has emerged as a subspecialty within ophthalmology, providing a bright future for patients seeking vision correction.









.jpeg)















.jpeg)






