Introduction
Cataract surgery is the most common ocular surgery performed in the United Kingdom (UK) with approximately 456,000 operations funded by the National Health Service from 2019-2020.1 Approximately 90% of patients who undergo cataract surgery achieve a postoperative visual acuity (VA) of 6/12 or better2; however, 2 to 14.7% of cases experience postoperative complications.3 One of these complications is Posterior Capsular Opacification (PCO), which occurs in roughly one in five eyes.4 This review will explore the aetiology, risk factors, prevention, differential diagnosis, referral criteria and current treatment of PCO.
Aetiology
To understand the aetiology of PCO, clinicians must first be familiar with the structure of the natural crystalline lens (lens). Hejtmancik and Shiels5 and Ruan et al have provided a detailed review of this.6
The human lens is an elliptically shaped transparent avascular structure. It consists of four main components depicted in Figure 1; namely, the capsule, epithelium, cortex and nucleus (from the outermost- to inner-most layers).7 During embryonic development the lens initially takes the shape of a sphere and is lined by lens epithelial cells (LECs).7 The LECs on the posterior surface differentiate and elongate to form the first lens fibers, after which all subsequent LECs differentiate at the pre-equatorial region. The lens fibres are then laid down in a concentric fashion to form the cortex (newest cells) and nucleus (oldest cells).7
‘Cataract’ is a term used to describe the opacification of the lens and is most commonly age-related, but can be associated with other factors such as diabetes, increased exposure to ultraviolet light, cigarette smoking, medical or illicit drug use, nutrient deficiency and ocular surgery.8 When the cataract impairs vision thereby limiting or restricting a patient from carrying out their daily routine, it is extracted by cataract surgery.
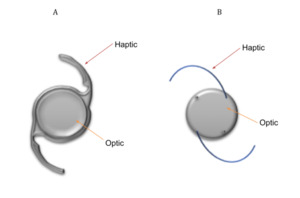
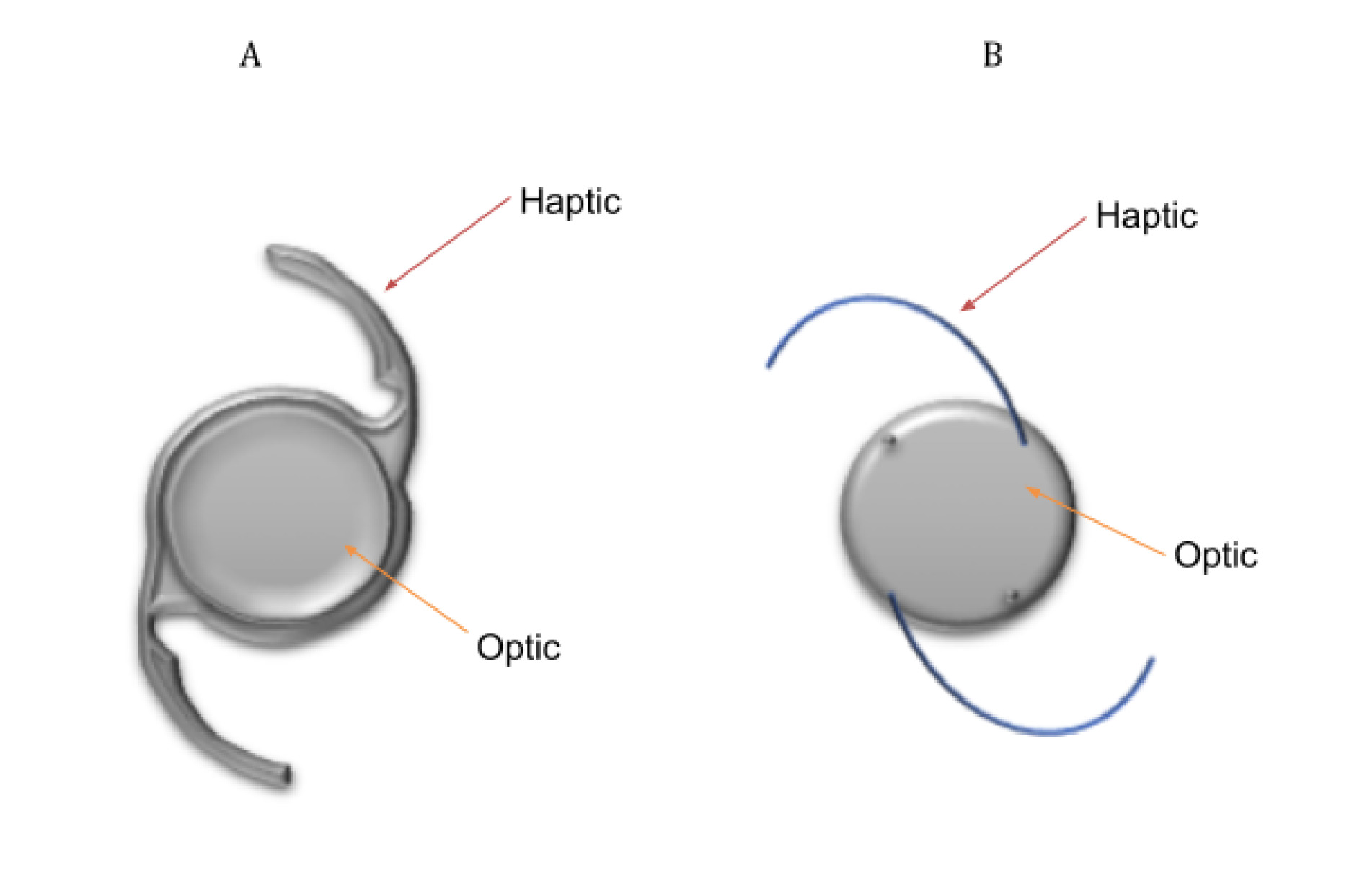
Extracapsular cataract surgery (ECCS) with phacoemulsification is the preferred method of lens extraction in developed countries (see Table 1 for steps involved in this type of cataract surgery).9 In this surgery, the central anterior capsule is removed by a procedure called capsulorhexis, but the posterior capsule and the peripheral anterior capsule of the lens are both kept intact.9 The residual capsular structure is called the ‘capsular bag’.9 It is important to note that the LECs that line the capsule are also preserved by this procedure. An intraocular lens (IOL) is implanted into the capsular bag to correct the patient’s ametropia. This is referred to as an ‘In-the-Bag’ IOL.10 One- and Three -piece IOL designs can be seen in Figure 2. The basic design of an IOL consist of an optic (this corrects vision) with side structures, called haptics, to hold the lens in place within the capsular bag.11
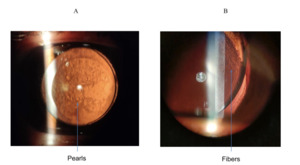
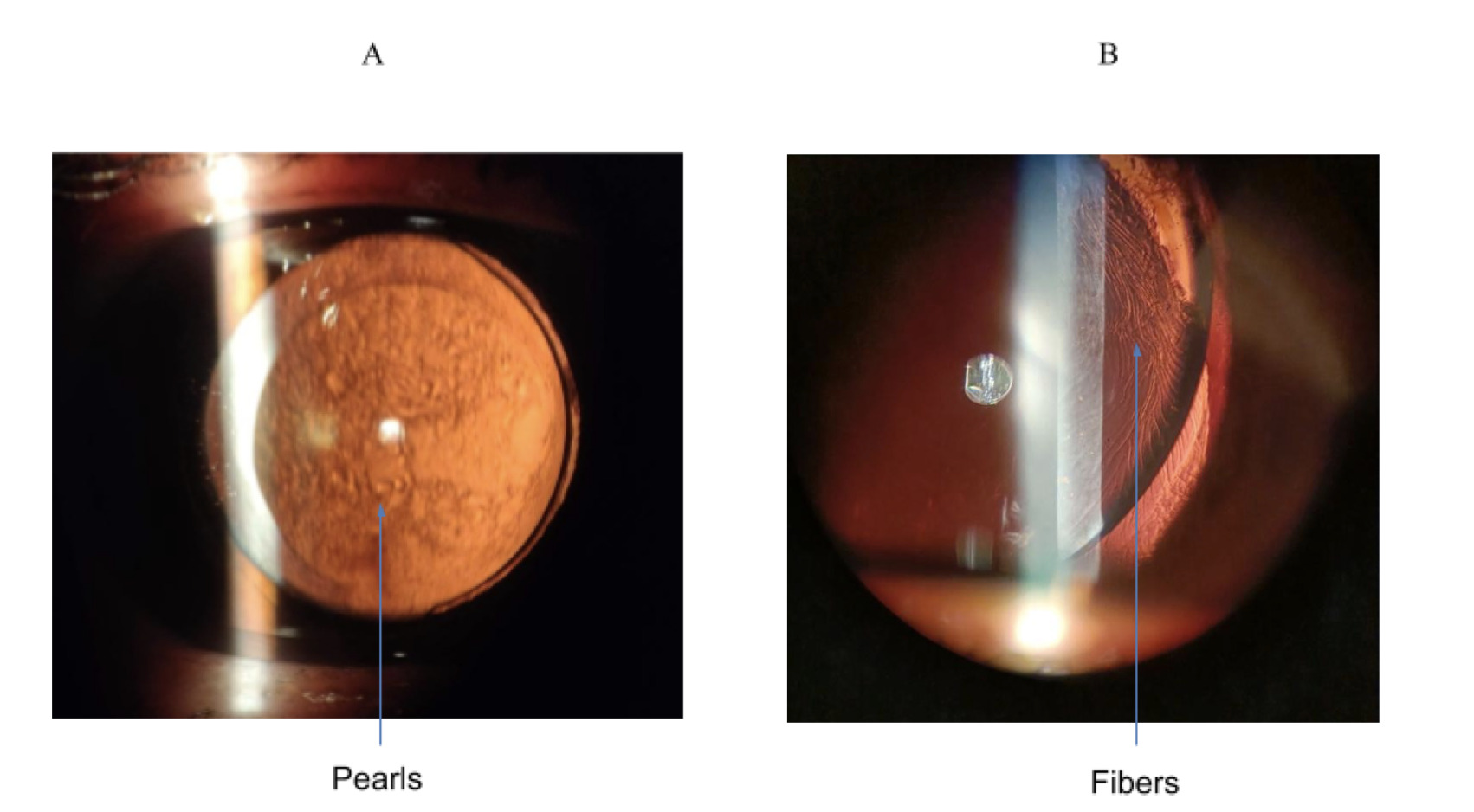
PCO is caused by abnormal differentiation, proliferation and migration of the anterior or pre-equatorial LECs along the posterior capsule, eventually clouding vision if the LECs cover the visual axis.15 An example of PCO is shown in Figure 3. In fact, this is typically seen through a slit-lamp under retro-illumination in a pearl or fibrous formation (see Figure 4). The pearl form is thought to be caused by proliferation of pre-equatorial LECs in an attempt to rejuvenate lens fibres,16 whilst the fibrous form is the result of LECs lining the anterior capsule which undergo epithelial-mesenchymal transformation (EMT).17,18
Risk Factors and Prevention
Surgical Technique
Any cortical material remaining in the capsular bag after ECCS is likely to trigger the formation of PCO. In view of this, cataract surgeons perform an enhanced cortical clean up by hydrodissection during ECCS.19 This involves separating the cortex and capsular bag (cortical cleaving) using fluid in multiple quadrants, which facilitates cortex and nucleus removal without zonal-capsular rupture.19,20 Any PCO resulting after a hydrodissection-enhanced cortical clean-up is likely to affect a smaller area of the central posterior capsule than if it was not performed.21
The Capsulorhexis is a critical step in ECCS, especially to help reduce the incidence of PCO.22 Constructing a circular Continuous Curvilinear Capsulorhexis (CCC) which is centred on the clinical approximation of the optical axis of the lens and has a diameter of 5.25 mm, enhances prevention of PCO and optimises the consistency of effective lens position and capsular strength.23,24 Furthermore, creating a Capsulorhexis which is marginally smaller than the diameter of the IOL optic size, encourages contact of the anterior capsule with the anterior surface IOL optic, thereby limiting or preventing migration of the LECs to the posterior capsule.19,25 Moreover, it essentially ‘shrink wraps’ the posterior capsular around the IOL so as to block off the interior contents of the capsular bag from surrounding aqueous humour and its’ associated post-ECCS inflammatory mediators,19,26 which will be discussed later in this review. The more experienced the surgeon, the better the Capsulorhexis,27 which helps to reduce the incidence of PCO.
Another surgical approach is Posterior Optic Buttonholing. This involves creating a posterior capsulorhexis and separating the posterior capsule and anterior vitreous hyaloid using an ophthalmic viscosurgical device, then finally entrapping the IOL optic in the posterior capsulorhexis opening. This blocks LECs from accessing the retrolental space thus preventing PCO. In addition, the posterior capsule is sandwiched between the anterior capsule and the IOL, preventing optic contact and thus fibrosis of the anterior capsule.28
The first Femtosecond laser -assisted laser surgery (FLACS) was performed in 200929 and has been proven to create a more precise corneal incision and Capsulorhexis, reduced effective phacoemulsification time, and allows for better IOL centration.29–31 Kovacs et al30 and Verdina et al31 found lower rates of PCO with FLACS after 18 months. This is supported by in vitro experiments; for example, Wertheimer et al did not notice any statistically significant difference in PCO in 18 cadaver eyes that underwent either FLACS, Phacoemulsification or ECCE.32
However, in a retrospective review, Rostami et al report an increased incidence of fibrotic PCO as early as three months post FLACS, but no cases of PCO were found in conventional ECCS with phacoemulsification.33 Similarly, when comparing the incidence of patients requiring laser treatment after ECCE or FLACs, Berk TA et al, reported that 5% more patients had to undergo laser treatment after FLACS than with ECCS alone.34 Further studies are required to clarify whether FLACS results in high rates of PCO.
Postoperative Inflammation
Any insult to the anterior capsule during surgery results in the breakdown of the blood-aqueous barrier and consequently an inflammatory response.35 This inflammatory response is constituted by the release of cytokines, growth factors and hepatocytes; notably, Transformation Growth Factor β (TGFβ) and Basic Fibroblast Growth Factors (bFGF) play a key role in the formation of PCO. Cataract surgery on rabbits has shown that the concentration of TGFB reduces post-surgery - this means bFBF and epidermal growth factor are unopposed and results in LEC proliferation – however, it returns to pre-operative levels by two weeks.36 TGFβ-2 is a subtype of TGFβ that is abundant in the aqueous humour37 in its’ inactive form, but is activated by plasmin proteinases such as Matrix metalloproteinases (MMPs).36,38,39 TGFβ is crucial in mediating the ‘‘wound healing’’ response in which LECs undergo EMT, resulting in the excessive deposition of extracellular matrix (ECM).40,41 In addition, it has been shown that TGFβ -2 increased collagen gel contraction and α–smooth muscle actin (α-SMA) expression in bovine LECs in a dose-dependent manner, whilst bFGF decreased these parameters.42 The upregulation of α-SMA is a biomarker for myofibroblasts.43,44 MMP -1 and MMP-2 have also been shown to increase after sham cataract surgery45 and MMP-2 plays a central role in the TGFβ -2 ECM contraction in the formation of fibrous PCO.39 Furthermore, Interleukin – 6 has also been shown to upregulate levels of TGFβ -2 and MMP-246,47 and αV integrins, specifically, αVβ8 integrin have been found to be critical for the onset of TGF-β–mediated PCO.48 In FLACS release of cytokines and growth factors in the early phase after cataract surgery, such as: Fibroblast Growth Factor - 2, leukemia inhibitor factor and Tumor Necrosis Factor -α could result in the proliferation of LEC’s and hence the development of PCO.49
Metformin has been shown to reduce the level of the EMT markers, α-SMA and pERK induced by TGF-β252 and administration of topical steroids post-cataract surgery reduces inflammation and is associated with lower rates of clinically significant PCO compared to treatment with nonsteroidal anti-inflammatory medications alone.50 Other Gene, Antineoplastic, Immunosuppressive and Cell Dissociation Solution therapies that target LEC signalling pathways, LEC proliferation and EMT have been proposed, but are not yet implemented in daily clinical practice as they need further robust clinical trials or adverse effects limit their practical application.48,51
Properties of an IOL
IOLs are made of one of three following materials: PMMA, Silicone and Acrylic. PMMA and Silicone IOLs have largely fallen out of favour for ECCS given that they both require a large corneal incision of >3.0mm to insert into the eye11 – PMMA IOLs are rigid43 and Silicone IOLs have thick haptics11 - and Silicone IOLs tend to have an affinity for bacterial adhesion52 but do not adhere to the posterior capsule.11 However, patients implanted with Silicone IOLs do show low rates of PCO.11
Acrylic IOLs are commonly used in ECCS in developed countries. There are two types of Acrylic IOLs: Hydrophilic and Hydrophobic.11 Hydrophobic IOLs are associated with lower rates of PCO compared to other materials, likely because of their sharp edge design and bioadhesive properties.53 When the body of the IOL meets its’ back surface to form a sharp edge – which is more likely to occur if the lens is unpolished during manufacture – this compresses and creates a sharp bend in the posterior capsule; hence, migrating LECs cannot travel any further to obscure the central posterior capsule. The Acrysof IOL is a Hydrophobic IOL that has better bio-adhesion to fibronectin than other IOL materials54,55 and fibronectin has been found attached to IOLs after cataract surgery.56 This means that bioadhesive materials like that of an AcrySof IOL can allow a single LEC layer to bond both to itself and the posterior capsule simultaneously, therefore sandwiching the LEC monolayer between the IOL and posterior capsule and reducing the likelihood of LEC migration and consequently minimises rates of visually significant PCO.19,55,57,58 This is called the Sandwich Theory. Lower rates of PCO also occur if a patient is implanted with an Acrysof IOL with an optic diameter is >6mm.59
On the contrary, Hydrophilic IOLs show higher rates of PCO than Hydrophobic IOLs.35,60,61 The difference in PCO rates may not be due to the material, but rather the edge design. Hydrophilic IOLs generally have a water content of about 26%, are normally vacuum packed before distribution and they are also rehydrated after being lathe cut in their dehydrated state causing the IOL to swell, which may change the edge design to a rounder profile thereby eliminating the barrier effect contributed by a sharper edge design.62 Nevertheless, the degree to which the composite material and its’ wettability affects PCO is controversial and clinical studies investigating the differences in hydrophobic and hydrophilic acrylic IOLs need to be done on larger cohort sizes.43
Similarly, regardless of the IOL material, if the IOL is placed in the capsular bag this produces a barrier effect to inhibit LEC migration and demonstrates lower rates of PCO than if the IOL were placed completely outside of the capsular bag or had one haptic in the capsular bag and one in the sulcus.10 In addition, the contact between the posterior surface of the IOL and posterior capsule can potentially be enhanced by using angulated haptics,63 but the barrier effect of a square edge design outweigh any differences in angulation in preventing PCO.64
Other promising and innovative methods of PCO prevention involving IOL design can found in Table 2. However, further human trials are needed to validate their efficacy.
Differential Diagnoses of PCO
Anterior Capsular Phimosis (ACP)
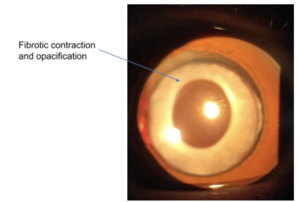
This is a fibrotic contraction due to the proliferation and differentiation of the anterior LEC’s when they touch the IOL, resulting in opacification of the peripheral anterior capsule (see figure 5).24,71 In order to prevent this, it is desirable that the Capsulorhexis is greater than 5.5mm19 and angulated haptics can be used to exert an affect by increasing clearance between the anterior capsule and the anterior surface of the IOL optic.72 ACP is treated by creating a break in the anterior capsule with Neodymium-doped Yttrium Aluminium Garnet (Nd:YAG) laser.71
Capsular Distention Syndrome (CDS)
This is a rare condition in which fluid accumulates in between the intraocular lens and posterior capsule (see Figure 6). The fluid is thought to originate from LEC products and becomes more opaque over time, giving it a milky appearance. It occurs when the anterior edge of the Capsulorhexis touches the anterior surface of IOL thereby trapping fluid in the capsular bag. Patients can be asymptomatic if it is late-onset and the fluid is clear or they may have a myopic shift. Risk factors include: retained OVD, insufficient sub-incisional cortical cleaning, IOL and the anterior capsular bag apposition and postoperative inflammation and IOL sequestration with Propionibacterium acnes. It is treated with Nd:YAG capsulotomy to allow fluid to drain away, but may require surgical intervention if Propionibacterium acnes is involved in the pathogenesis.73
IOL Glistening
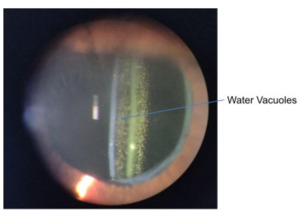
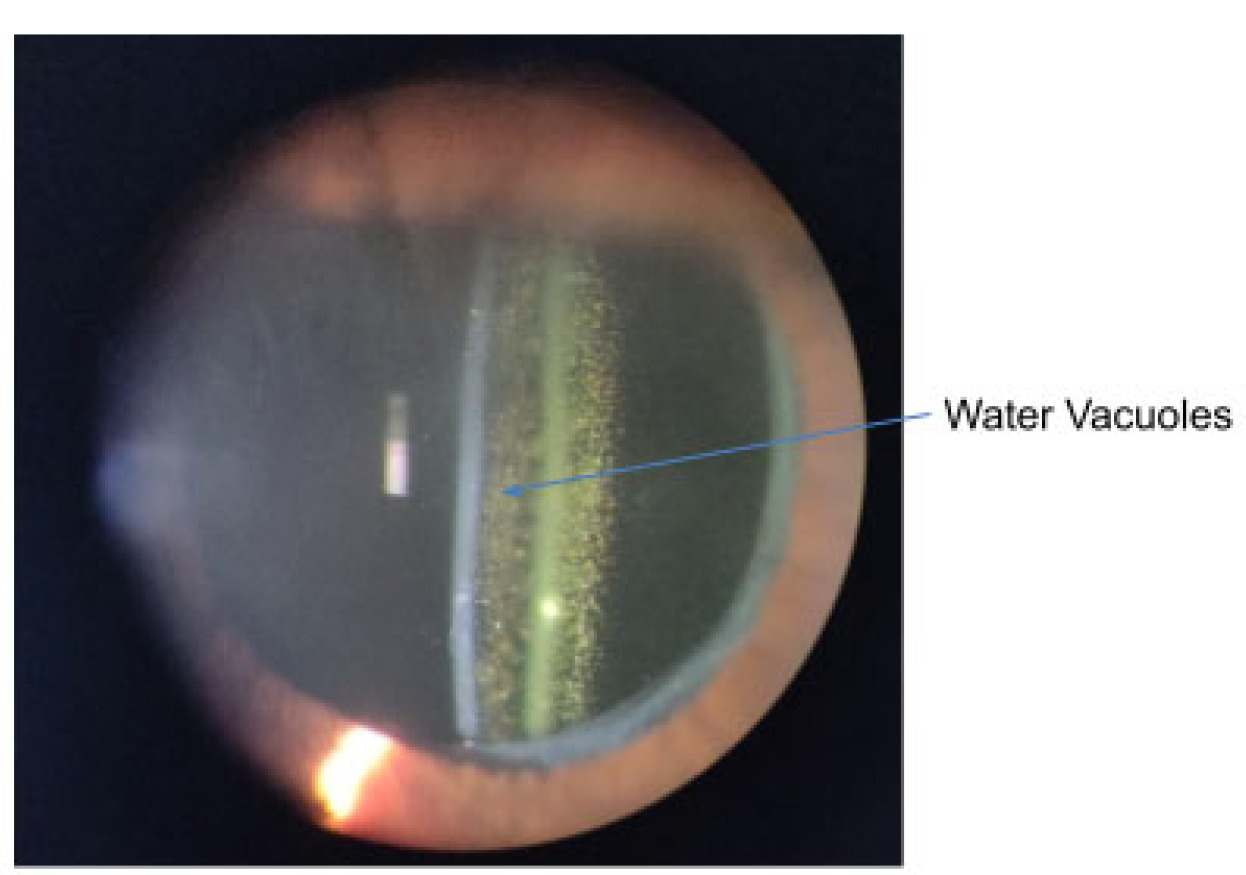
This occurs predominately in hydrophobic acrylic IOLs. They are small water-filled vacuoles that form when water permeates through micro-channels in the lens.74 These vacuoles cause light scatter and a reduction in contrast sensitivity which can impair vision, mostly due to glare.75 If the glistening is dense enough (see Figure 7) to disrupt the patient’s vision, an IOL exchange will be required to restore functional vision.11,76
Soemmering’s Ring
Soemmering ring has a donut-like shape and is triggered by extruded cortical material when the anterior capsule is ruptured; after ECCE, it is the equatorial LEC’s that proliferate and migrate to the posterior and residual anterior capsule to form Elschnig’s pearls.19
IOL Opacification
This is a rare, irreversible, late-onset opacification that occurs in Hydrophilic IOLs.77 There are two types of opacification. In type 1, calcium precipitates on the surface the IOL; and in type 2, calcium deposits within the substance of the IOL optic.75,78 Risk factors include diabetes mellitus, glaucoma, uveitis, postoperative inflammation, and intraocular calcium concentration.77,78 The only treatment option is IOL exchange, which is unfortunately associated with high rates of complications such as a rise in intraocular pressure, retinal detachment and corneal decompensation.79
Interlenticular Opacification
This type of opacification occurs in dual-optic or Piggyback IOL Systems. A dual-optic IOL system is characterised by two IOLs implanted into the capsular bag,80 whilst Piggyback IOL System features one IOL in the capsular bag and the other place in the sulcus.81 The opacification occurs between the two IOLs in both systems by the same mechanism as PCO80,82 and can be treated with Nd:YAG Capsulotomy.83
Referral Criteria
Before making a referral for the treatment of PCO, clinicians must consider three factors: VA, symptoms caused by PCO and the degree of PCO.
Patients suffering from PCO often experience reduced/misty vision, glare or both – similar to symptoms reported by patients with a visually significant cataract. It is important to note that VA alone does not reflect the extent of the patient’s subjective visual disability and we must consider contrast and glare sensitivity, stereoacuity, and visual fields which are independent risk factors of visual disability.24,84 Lu et al noted that patients with slight PCO and good VA (0.1 LogMAR or better) still had notable subjective disability; hence, we must account for the impact of PCO in the patient’s quality of life and their ability to carry out daily routine tasks in these circumstances.85 Of note, the pearl formation of PCO causes a greater reduction VA and contrast than the fibrous form.86
There are several systems used to assess the severity of PCO,87 but it is commonly examined on the slit lamp under retroillumination and I will therefore discuss grading systems pertaining to this mode of assessment.
Kruger et al used a grading system that identified PCO by its pearl or fibrosis morphology and graded Anterior Capsular Opacification (ACO).88 The capsule behind the optic was evaluated within a central area measuring 3 mm in diameter and evaluated in the periphery.88
Similarly, Aslam et al report another slit-lamp based grading scale adopted by many clinicians.88 The four-point grading scale distinguishes between fibrosis and Elschnig pearl formation. Preferably, this grading scale should be used in daily clinical practice going forward as it gives details about the location of the PCO and its severity. A comparison of the grading systems can be found in Table 3.
The guidelines for referring PCO vary between ophthalmology services, so it is important to familiarise yourself with your local guidelines. A direct referral means that patients will be seen two weeks sooner for treatment than if they were referred via their general practitioner.90 The Royal College Of Ophthalmologists (RCOphth) prefers PCO to be referred based on presence of characteristic signs visible on slit lamp examination and patient symptoms as they feel this criteria outweighs referral based solely on visual function given that the severity of PCO correlates poorly with high-contrast VA, and blurred vision, glare, dysphotopsia and reduced contrast. In view of this, RCOphth do not advise relying solely on VA threshold for the referral of PCO.91 However, the referrals will normally be made to the hospital eye service or a community ophthalmological clinic for one more the following reasons92:
-
If visual acuity has dropped from 6/6 or better to 6/9 or less with capsular thickening present and no other clinical reason for the visual loss detectable.
-
Patient self-reports loss of distance or near visual acuity in the operated eye and capsule thickening is the probable diagnosis.
-
Where other pathology is present, but you consider a significant loss of visual acuity is due to capsule thickening.
-
The patient wishes to be referred.
Treatment
Nd:YAG laser capsulotomy is typically used to treat adult PCO, albeit, paediatric cataract surgeons prefer performing a posterior capsulotomy with or without anterior vitrectomy in young children or those with developmental delay, as these patients are often unable to sit still for the procedure, and the associated PCO is normally more dense than that of adults.24,93
The incidence of Nd:YAG capsulotomy after cataract surgery ranges between 2.4-12.6% at 3 years and 5.8-19.3% at 5 years.58 To perform Nd:YAG Capsulotomy, a patient’s eye is anesthetised and a contact surface lens is placed on it whilst they are on the slit lamp; the clinician then focuses several pulses of laser shots on the central posterior capsule in a circular, cruciate, horseshoe or spiral pattern94 to create a large enough opening in the visual axis, so that the patient can see clearly again. Complications of Nd:YAG capsulotomy include raised intraocular pressure, anterior uveitis (Iritis), IOL pitting, cystoid macular oedema, retinal detachment, disrupted anterior hyaloid surface and IOL dislocation.94,95
Conclusion
PCO is a common compilation after cataract surgery despite the intraoperative precautions taken to limit its’ occurrence. Current research on preventing PCO is promising, but its’ practical application is limited by the need for robust randomised human clinical trials to study the adverse effects of some therapies. Optometrists are likely to be the first clinicians to evaluate and diagnose this condition, and should be able to recognise and differentiate PCO from other pathology so that patients receive appropriate treatment - Nd:YAG Capsulotomy or posterior capsulotomy - as soon as possible so as to minimise the impact on their quality of life.