Introduction
This paper describes a case report of an asymptomatic patient that presented for a routine eye exam with the subsequent detection of a suspicious choroidal lesion leading to the ultimate diagnosis as a choroidal melanoma. Critical detection of this life and sight-threatening disease on a routine eye exam allowed for prompt treatment and management. No identifiable health information was included in this case report.
Case Report
A 58-year-old Caucasian female patient presented for a comprehensive eye exam reporting mild distance blur, gradually worsening, and slightly worse in the right eye than the left eye. Pertinent medical history included coronary artery bypass surgery, history of throat cancer, insomnia, and a history of a stroke more than 5 years ago. Pertinent ocular history included glaucoma suspicion with subsequent glaucoma testing revealing no signs of glaucoma and was kept on yearly observation.
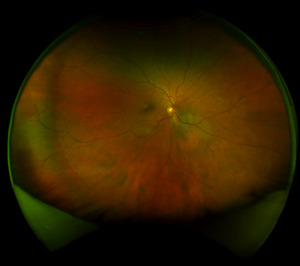
Best corrected visual acuity was 20/20-3 right eye and left eye. Anterior segment was unremarkable in both eyes and intraocular pressure was normotensive at 19 millimeters of mercury in the right eye and 20 millimeters of mercury in the left eye. On dilated fundus exam trace nuclear sclerosis was only noted in the right eye. The optic nerve’s cup-to-disc ratio was measured as 0.35 horizontally and vertically in the right eye, 0.30 horizontally and vertically in the left eye. Both optic discs were well perfused without any abnormalities. The macula was flat in both eyes. In the right eye, there was a faint, variably pigmented lesion 4-disc diameter in size that was located 0.5-disc diameters inferior nasal to the optic nerve extending into the inferior nasal quadrant as noted in Figures 1 and 2.
The lesion itself had orange patches of possible increased visualization of choroidal vasculature versus possible lipofuscin. A separate, less suspicious choroidal nevus was noted in the far inferior nasal periphery of the right eye that measured 4-disc diameters in size and was noted to be flat without lipofuscin nor subretinal fluid and with an isolated drusen at the edge of the nevus. Given the first choroidal nevus’ proximity to the disc, size, and suspicion of lipofuscin, the patient was referred to a retinal oncologist for further workup and management. The patient saw the retinal oncologist one week later where on dilated fundus exam the lesion was described as a predominantly amelanotic choroidal lesion with orange pigment, drusen and presence of subretinal fluid.
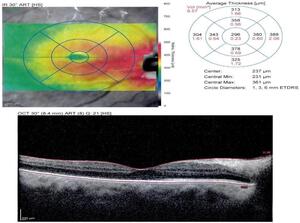
Multiple imaging techniques were utilized to better ascertain the type of choroidal lesion. Optical coherence tomography of the macula revealed retinal elevation consistent with subretinal fluid and focal retinal pigment epithelium elevation consistent with drusen as seen in Figure 3. Enhanced depth imaging optical coherence tomography noted an optically hyper-reflective lesion measuring 2.0 millimeters with overlying subretinal fluid and retinal pigment epithelium level deposits.
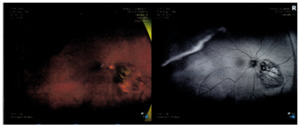
Fundus autofluorescence yielded hyper-fluorescence associated with the pigmented lesion along with hypo-fluorescence associated with subretinal fluid, and predominantly hypo-fluorescent retinal pigment epithelium changes overlying the lesion (Figure 4). B-scan noted a dome shaped hyper-reflective choroidal lesion measuring 7.6 by 6.2 millimeters in size and was 2.6 millimeter thick. There was low to medium internal reflectivity on the A-scan.
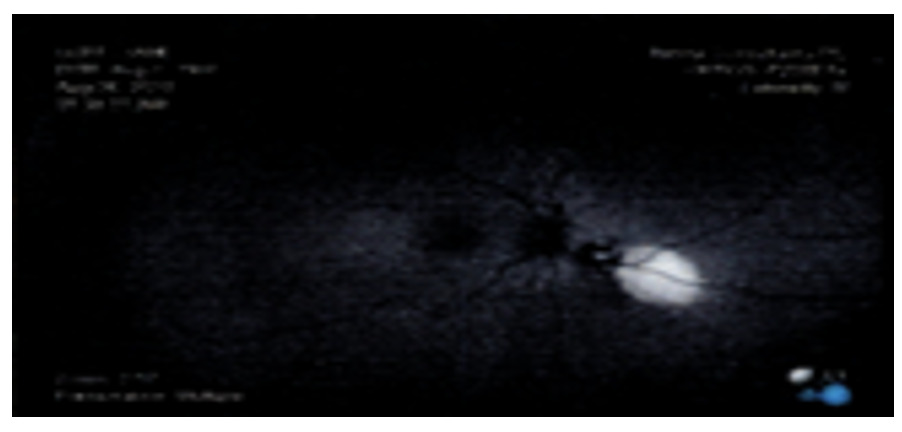
As seen with Figure 5, fluorescein angiography revealed normal arteriovenous transit with hypo-fluorescence consistent with the choroidal lesion. With Indocyanine green angiography, hypo-cyanescence was seen correlating with the choroidal lesion.
Upon completion of the work up the patient was diagnosed with a presumed small choroidal melanoma in the right eye due to the thickness being greater than 2 millimeters, presence of lipofuscin, proximity to the disc, and subretinal fluid. The patient was scheduled for a biopsy to determine the malignancy potential of the lesion. The biopsy revealed a malignant class 2+ melanoma with confirmed BAP1 mutation. The retina oncologist recommended and performed a surgical placement of a radiation plaque over the tumor as seen in Figure 6.
Further systemic workup of the lungs and liver did not reveal any metastasis to those organs. Initially, the tumor was regressing smoothly, however; the patient eventually developed a proliferative vitreoretinopathy in the right eye. Methotrexate injections were done in the beginning as treatment to prevent a recurrent retinal detachment. The injections were discontinued four weeks later due to the development of an exudative retinal detachment secondary to the proliferative vitreoretinopathy. Eventually the decision was made for secondary enucleation when the vision worsened to hand motion due to poor visual potential. Enucleation was done without complication and ultimately the patient was fitted with an ocular prosthesis.
Discussion
Choroidal melanomas are the most common primary intraocular malignancy in adults and represent 80% of all uveal melanomas.1,2 Risk factors for choroidal melanomas include fairer skin, lighter iris color, prolonged sunlight exposure, and diagnosis after the age of 60 years.1,3 The lesion can present with a collar-stud shape on fundus examination due to the lesion penetrating Bruch’s membrane.1,2 Metastasis is rare only occurring in 1-2% of patients with detectable metastasis commonly occurring in the liver, bone, and lung.1 Mortality is significant at 50% at 10 years for metastasized choroidal melanomas.1 Several histological features can be associated with a poorer prognosis, including a larger number of epithelioid cells, miotic activity, larger tumor dimensions, certain tumor genetic features, extrascleral extension and anterior location of the lesion.1–3 An example of a higher risk tumor genetic feature is the somatic mutation in tumor suppressor gene BAP1 as present in this patient.1,3,4 BAP1 mutations are seen in close to 50% of uveal melanomas and signify a greater chance of metastasis.1,3,4
There are typically no symptoms with a choroidal melanoma and can often be detected on routine funduscopic examination.1,2 Symptoms of vision loss usually occur when the tumor encroaches onto the fovea, an exudative retinal detachment involving the macula occurs, or the tumor contacts the lens therefore early choroidal melanoma not involving the macula can go undetected by the patient.5 Approximately 60% of tumors are located within 3 millimeters of the optic disc or fovea.1 Common associated characteristics include a solitary elevated subretinal grey-brown or amelanotic domed or collar button shaped mass, presence of orange pigment clumps, associated hemorrhages and subretinal fluid.1,2,5,6 Other possible signs include sentinel vessels, choroidal folds, inflammation, rubeosis iridis, secondary glaucoma and cataract.1
The main differential for a choroidal melanoma is a choroidal nevus which can be present up to 6% of all adult Caucasian eyes.6 Choroidal nevi are typically an oval or circular in shape, brown, grey or amelanotic lesion with indistinct margins, overlying drusen, and depigmented halo.1,2,6 When surface drusen are present on a nevus this is indicative of chronicity. With choroidal nevi there is typically little to no orange pigment and commonly presents without a serous retinal detachment.1,2 Though lipofuscin is typically associated with choroidal melanomas, few cases have been reported of choroidal nevi with areas of orange pigment and serous detachment present.7–11 Choroidal nevi are present in 5-10% of Caucasian eyes and less commonly seen in darker skinned individuals.1,6 A majority of choroidal nevi growth occurs pre-puberty, therefore visible growth should be a cause of concern for malignancy in adults.1 Similar to choroidal melanomas, most choroidal nevi are symptomless and are detected on routine exams. However, a study by Shields did find that 10% percent of patients with a sub-foveolar choroidal nevus experienced visual acuity decline as a symptom especially with overlying pigment epithelial detachment and foveal edema.7
Even if a choroidal nevus is detected, there is a small chance of conversion to a melanoma. A common mnemonic for differentiating choroidal melanomas and nevi is TFSOM-DIM or To Find Small Ocular Melanomas Doing Imaging.12 TFSOM-DIM encapsulates the risk factors for a choroidal melanoma as: Thickness, if equal or greater than 2 millimeters on ultrasound; Fluid, located subretinal on optical coherence tomography; Symptoms, of vision loss or photopsias; Orange pigment, on autofluorescence; Melanoma hollow, on ultrasound; Diameter, equal to or greater than 5 millimeters on photography (Table 1A).12,13 Another common mnemonic is TFSOM-UHHD or To Find Small Ocular Melanoma Using Helpful Hints Daily.13 This mnemonic labels the risk factors as: Thickness over 2 millimeters; Fluid; Symptoms; Orange pigment; Margin within 3 millimeters of the optic disc; Ultrasound hollow; Halo absent; Drusen absent (Table 1B).13,14 The estimated nevi conversion to melanoma risk over 5 years was found to be 1% with zero risk factors, 11% with one risk factor, 22% with 2 risk factors, 34% with 3 risk factors, 51% for 4 risk factors, and 55% for 5 risk factors.12
Other differential diagnoses for pigmented lesions include melanocytomas, choroidal neovascularization and retinal artery macroaneurysms. Melanocytomas present as a deeply dark pigmented lesion typically located at the optic disc. This finding is a congenital hypertrophy of the RPE that presents with a well-defined margin, possible presence of lacunae, and is often grey black in color.1 In addition to a “pigmented” appearance these can also present with fluid and hemorrhage in the subretinal or suprachoroidal space similar to choroidal melanomas.1
Differential diagnoses for an amelanotic melanoma include a circumscribed choroidal hemangioma, that presents with a posterior pink, smooth dome shape; solitary choroidal granuloma, as seen in sarcoidosis and tuberculosis; a large elevated eccentrically located choroidal neovascular lesion; posterior scleritis, when presenting with a large, elevated lesion; and a prominent vortex vein ampulla.1
Diagnostic testing is critical to the differentiation of choroidal lesions. Ultrasound is very useful for measuring the lesion dimensions and to detect lesions through opaque media.1,6 In addition, choroidal melanomas will have a unique characteristic when B-scan ultrasound is performed. Choroidal melanomas will typically have internal homogeneity with low to medium reflectivity, choroidal excavation, and orbital shadowing.1,2,6 Due to the internal homogeneity of the lesion it is typically referred to as acoustically hollow or as having a basal quiet zone.1,2,6 If a collar-stud configuration is present when imaged this is considered pathognomonic for a choroidal melanoma.1 Fundus autofluorescence is another useful diagnostic test where a choroidal melanoma will present with intense diffuse or confluent hyper-fluorescence.1,2 An optical coherence tomography of the macula can aid in the detection of subretinal fluid before it becomes clinically detectable on funduscopic view.1,2 Lastly, a biopsy is beneficial when the diagnosis cannot be established by less invasive methods.1,2 Genetic tumor analysis is becoming more vital in the management particularly regarding prognosis as metastasis occurs almost exclusively with certain genetic profiles.1 Therefore, increased observation could be advantageous for patients where the melanoma profile reveals a high risk of metastasis.1
It is also important to be aware that choroidal melanomas can be either a primary tumor or a secondary tumor, a result of metastasis. Due to this, additional systemic testing is necessary. This testing will be directed towards locating the primary tumor or ruling out potential metastatic spread. Common systemic testing includes liver function testing and ultrasonography.1 Treatment and management is customized based on the characteristics of the particular tumor and patient characteristics such as general health, age, state of the fellow eye, and patient preferences.1 There are various treatment options available; the first line option is brachytherapy, which is when a plaque is sutured to the sclera for a few days.1,5 This treatment is useful for tumors less than 20 millimeters in basal diameter and up to 10 millimeters in thickness.1 Regression commonly begins 1-2 months post treatment and continues over several years eventually leading to a flat or dome shaped pigmented scar. Complications of this treatment include cataract, papillopathy, vitreous hemorrhage, neovascular glaucoma and radiation retinopathy.1,5 A second treatment option is external beam radiotherapy, which uses high dose fractionated irradiation in the tumor with relative sparing of adjacent tissues and is typically used for tumors deemed unsuitable for brachytherapy due to a large tumor size or posterior location of the lesion.1,5,12 External beam radiotherapy has been shown to be effective in choroidal melanomas with basal diameter of at least 18 millimeters and thickness of 8.7 millimeters.15 Survival is comparable to radiotherapy but regression is slower with external beam radiotherapy with similar intraocular complications.1 A third treatment option is stereotactic radiotherapy. This treatment uses multiple collimated beams from different directions so only the tumor receives a high dose of radiation and is mainly used for tumors not suitable for plaque radiotherapy.1 This allows a more accurate target of treating tumors especially those near the optic disc and macula.13 Multiple types of stereotactic radiotherapy exists that have been used effectively on tumor sizes with a basal diameter of 10.3 millimeters and thickness of 8.7 milimeters.16 The fourth treatment option is transpupillary therapy, which uses an infrared laser beam to induce tumor cell death by hyperthermia rather than coagulation usually for small tumor when radiotherapy or brachytherapy is considered unsuitable.1,12,13 Transpupillary therapy is typically done with tumor thickness less than 3 millimeters.1,13 Complications include retinal tear formation, vascular occlusion, neovascularization, and retinal traction.1,13 In addition to causing regression of the tumor another important goal of treatment is to prevent the development of a painful and cosmetically unappealing eye, while conserving useful vision as much as possible.1 As a result, treatment with enucleation is used as a last resort, for when the tumor size is large with thickness greater than 10 millimeters and basal diameter greater than 18 millimeters, signs of disc invasion, extensive involvement of the ciliary body or angle, irreversible loss of useful vision and poor motivation to keep the eye.1,5,13 Lastly, systemic chemotherapy is reserved for metastatic melanoma and has not been shown to be beneficial in non-metastatic choroidal melanoma cases.1
In this patient’s case, given the poor vision remaining after the retinal detachment, retinal detachment surgery might have improved her vision temporarily. However, it was likely that the vision would continue to decline due radiation damage to the optic nerve and the retina. The patient declined intervention to repair the retinal detachment and wished to pursue enucleation to reduce her risk of metastasis from the choroidal melanoma. If the choroidal melanoma had been detected earlier when it was smaller, then potentially less radiation damage to the retina would have occurred. This could have resulted in a lower risk for complications including the development of a proliferative vitreoretinopathy and exudative retinal detachment thereby preserving the vision.
Conclusion
Choroidal melanomas are a treatable condition; however, if not diagnosed in a timely manner, they can be vision and even life-threatening if metastasis occurs. In this case, the patient presented for a routine eye exam reporting that they were not experiencing any concerning symptoms. During the exam all ocular findings were normal up to the dilated fundus exam where a presumed choroidal melanoma was detected. Performing a dilated fundus examination was critical to the identification of the lesion and allowed for timely referral, treatment and management, which is important in decreasing the mortality risk of the patient.
Disclosures
The authors have no financial or proprietary interest in any material or method mentioned in this article. This article has been peer reviewed.