Introduction
Optic nerve head drusen (ONHD) may appear clinically as crystalline, hyaline-calcified, lobulated aggregate bodies within localities of the optic nerve. Nonetheless in the case of buried ONHD the drusen bodies may not be as visible, but the optic nerve may have a swollen edematous appearance. In this instance although the nerve margins may be blurred, the adjacent vessels will not be obscured as is the case for true disc edema. Optical Coherence tomography (OCT) is useful at visualizing the drusen deposits and illustrates the bump typical appearance of ONHD. Similarly, ultrasonography may demonstrate the presence of buried drusen. However, OCT alone may not be conclusive at differentiating ONHD from mild papilledema.1–3
Ocular damage seen with ONHD may arise from direct physical compression, circulatory vascular insufficiency and anomalies affecting the axoplasmic flow. Anomalies of superficial capillary circulation, vessel density and low blood flow give support to a vascular theory of ocular impairment in ONHD. Hyperreflective ovoid shaped formations located peripapillary in ONHD have been suggested to represent piling and swellings of axons. Alteration of the homeostasis of axoplasmic transport system has been suggested to give rise to the accumulation of hyaline in ONHD. A disorder of axoplasmic flow metabolism rather than an anomalous axoplasmic transport has also been proposed to give rise to hyaline accumulation in ONHD1–5
Data exists regarding the rate of VF (VF) and RNFL (RNFL) progression.1,3–5 VF loss occurs regularly, may be stable or progress slowly, tends to worsen with increasing age and is more severe with discernable ONHD. VF loss may range from localized pocket defects to overall constriction.6 Using a modified Esterman grid technique, Lee and Zimmerman estimated a rate of progression of approximately 1.58%/year during a 36-month period.4 They suggested that the rate of progression of VF loss may correlate with increasing age and applicable to patients that develop progressive VF loss. Although this technique is not comparable to automatic static perimetry, the results from Lee and Zimmerman provided one indication of a rate of progression. Estela et al. provided a rate of VF progression based on mean deviation values.6 They indicated that while most eyes had a slow rate of progression, older subjects and those having more severe VF loss had faster rates. In eyes with moderate progression, the rate of progression was between -0.5 dB/year and -1 dB/year. The faster rate of progression was -1.19 dB/year. It is worth noting that although the pathological processes that give rise to VF loss in ONHD are different from that of glaucoma, the rate of VF decline in glaucoma using a similar technique as Lee et al. was 1.3% per year.4,6,7 It is also approximately equal to that of Estela et al. This suggests that in those cases that develop VF loss in ONHD a threshold may be reached whereas a programed decline occurs that parallels that of glaucoma.
A correlation between VF loss and retinal nerve fiber loss has been documented. This correlation is more factual in cases of visible drusen.1 Less data exists regarding the rate of progression of RNFL loss in ONHD. While Pilat and associates indicated significant thinning for the RNFL, no estimation of the rate of progression was provided.4
The asymmetric VF and RNFL loss with the presence of a relative afferent pupillary defect (RAPD) in this case, may provide an insight into the inter-relation between structure and function that may occur in some cases of ONHD and is currently missing for this condition.
Case report
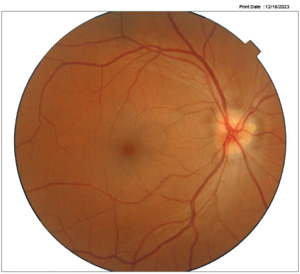
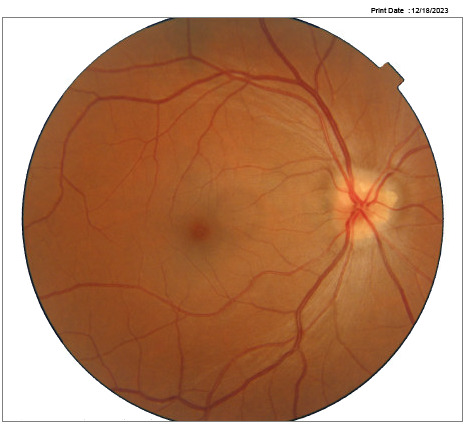
A 45-year-old female reported unremarkable health and no use of medications. The best-corrected visual acuities were 6/6 -2 in the right eye and 6/6-1 in the left eye. Preliminary examination findings and slit lamp examination of the anterior segment were unremarkable for both eyes. A left eye RAPD was observed using the swinging flashlight technique. Applanation tonometry measurements during varied visits range between 12-16 mm Hg. The dilated fundus exam revealed optic nerve head drusen in both eyes (figure 1a-b).
The right eye VF (Zeiss Humphery VF analyzer 3) result was normal with a mean deviation of -1.48 dB and a pattern standard deviation of 1.38 dB. The left eye VF revealed diffuse defects with a mean deviation of -9.38 dB and a pattern standard deviation of 6.65 dB. The difference between eyes for the mean deviation was 7.9 dB (figure 2a-b).
The probable progression to reach the level of mean deviation loss of the left eye assuming a linear rate of progression of 1%/ year was 7.9 years from the date of the baseline VF.5 The optical coherence tomography (RTvue-100) values were within normal limits for the right eye. RNFL loss was evident in the left eye with values outside normal limit (average 83 um, superior 90 um, and inferior 76 um). The percentage average, superior and inferior RNFL loss between the two eye were 21%, 13% and 30% respectively. The left eye ganglion cell complex had borderline values for the inferior (85 um), focal loss volume percentage (2.09%) and global loss volume percentage (7.38%) (figure 3).
Estimation of the rate of progression of the average RNFL, assuming a 7.9-year period (21um/7.9 years) it will be 2.65% um/year. The relative rate of loss of RNFL average/mean deviation VF was approximately 2.65 um/dB loss. The average RNFL value of the right eye was 104 um. The estimated um loss in one year using a 2.65% um/year loss (104um x .0265%) will be approximately 2.75 um.
Discussion
Asymmetric optic nerve pathology provides a unique opportunity to analyze the relative morbidity and consequent occurring clinical signs. An asymmetric presentation may suggest that the less affected eye is at risk of progressing to a magnitude comparable to that of the more affected eye. Information of rate of progression is limited for optic nerve head drusen.1,3–6 Lee and Zimmerman reported a rate of VF loss progression of approximately 1.58%/year.4 Estela et al. using mean deviation values indicated a rate of progression ranging from -0.5 dB to -1 dB/year.6 Both researchers implied that this faster rate progression applied to older subjects and those that develop progressive or moderate VF loss. Mustonen and Nieminen reported progression of VF and thinning of RNFL but did not detail time span of its occurrence.8,9 The RNFL thinning has been described to correspond to the VF loss and to the nasal location of the aggregated drusen.1 To our knowledge, only one study quantified RNFL loss in optic nerve head drusen. Pilat and associates reported thinning of the RNFL but did not provide a rate of progression for the RNFL.5
It is acknowledged there are limitations in predicting a rate of VF and RNFL loss progression, by applying the limited data available to this sole case. It is also recognized that in ONHD, the progression of VF loss is sporadic and may only apply to severe cases and older age. Nonetheless, an estimate using a rate of progression of 1%/year degree of loss was assessed to occur in 7.9 years from the baseline VF. This rate of progression was selected since it used mean deviation values as in this case. Acknowledging that ONHD is an untreatable condition, the value of estimating the years of conversion rests in predicting a rate of retinal nerve fiber loss. Similarly, although an association between structure and function has been documented for ONHD, this correlation may be less in cases of buried drusen.1 Taking this into consideration nonetheless, the rate of loss for the RNFL average/mean deviation VF was estimated to be 2.65 um/dB loss in this case. In glaucoma the mean rate of mean deviation VF progression has been documented to be -0.80 dB/year.10 Miki and associated reported a rate of global RNFL in eyes that develop VF loss to be -2.02 um/year.11 The loss ratio of RNFL in um to mean deviation in dB for glaucoma using these researchers’ values would be (2.02 um/year/0.80 dB/year) approximately 2.52 um/dB. This is comparable to the loss ratio of RNFL average in um to mean deviation in dB estimated for this case. This suggests that similar rates of progression as in glaucoma may occur in a subgroup of ONHD patients.
The estimated um/year loss in this case compared to that documented for eyes that develop VF loss in glaucoma (-2.75 um/year/-2.02 um/year) was 1.4 times more. While it appears that RNFL loss may occur faster in ONHD than glaucoma, it must be reiterated that these values were estimated from the rate of documented VF loss for severe and older cases of ONHD. Since the estimated loss ratio of RNFL in microns to mean deviation VF in dB loss in this case and that calculated for glaucoma were comparable, it is more likely that the actual RNFL loss in this subgroup of ONHD approaches that of glaucoma. These observations may indicate that once a threshold level of damage is reached in a subgroup of OHND patents, structure-function loss may occur as in glaucoma.
A RAPD was observed in this case, although it was not quantified using neutral filters. The mean deviation VF difference of 7.9 dB corresponded with the documented level of mean deviation difference loss between eyes that may manifest a RAPD.12 Its presence also corresponded to the RNFL percentage difference loss between eyes that may result in a RAPD documented by several investigators.13–16 Tatsumi et al. indicated that a 0.6 log unit RAPD was associated with roughly 27% relative RNFL loss.14 About 17% relative RNFL loss was documented by Chew et al. to be related to a RAPD range of 0.3 to 0.9 log units.15 A 23% loss related to a RAPD of 0.6 log units was documented by Nakanisshi et al. for patients having a variety of optic neuropathies.16 The percentage difference for the average and inferior RNFL loss (21% and 30% respectively) in this case of ONHD was within the variance range for the documented presence of a RAPD. This suggests that the manifestation of a relative afferent pupillary defect in a subgroup of ONHD cases may correspond to the degree of differential RNFL loss observed for other optic nerve pathologies.
Currently there is no treatment available for ONHD. Although controversial, some investigators have indicated that lowering the intraocular pressure (IOP) may be beneficial in cases of ONHD with ocular hypertension. Since the blood flow at the optic nerve arterioles may be affected in ONHD, a vascular etiological mechanism analogous that responsible in glaucoma may also occur in ONHD.4,17 Their indications were based on their observations of ONHD patients undergoing hypotensive therapy demonstrated less VF loss and progressive optic nerve damage compared to those untreated.3 Nolan and associates however, suggested that in normo-tension subjects with ONHD, lowering IOP may not have an effect at slowing damage to the optic nerve.3,18 In this case, IOP lowering medications were not considered as the IOP in the patient were low and the benefit of treatment remains contentious.
Conclusion
In summary the asymmetric loss of VF and RNFL loss in this case of ONHD parallels to documented loss associated in glaucoma. Although the inference attributed in this case may not apply in general to ONHD, in specific cases involving older age individuals with severe structural/functional loss, it may follow an apoptosis process that generally may be analogous to that of glaucoma.