Introduction
Routine eye exams can discover common findings that imitate more serious eye diseases. A patient displaying true disc edema and pseudo disc edema is rare. This case report will elaborate on both the commonly found disc drusen and the less commonly seen disc edema associated with amiodarone use.
Case Report
A 64-year-old white male presented to clinic for a full, diabetic eye exam. His chief complaint was that allergies were bothering him and causing his eyes to water. The patient had no noteworthy ocular history and the only significant medical diagnosis included cardiac arrythmia, which he was treated with amiodarone.
The patient’s entering uncorrected acuities were 20/20-1 OD, 20/25-1 OS. Preliminary testing revealed full and unrestricted EOMs, with normal pupils, and confrontational fields in both eyes.
Anterior segment displayed mild dermatochalasis affecting both upper lids, blepharitis, and meibomian gland dysfunction. Mild superficial punctate keratitis was also seen bilaterally. Anterior chambers were deep and quiet with intraocular pressures of 19 mmHg in both eyes taken by Goldmann tonometry.
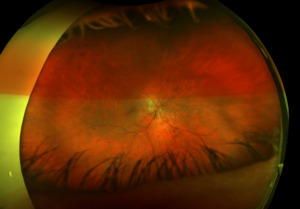
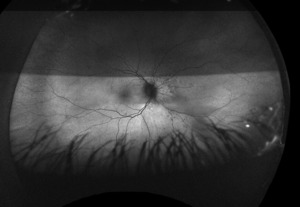
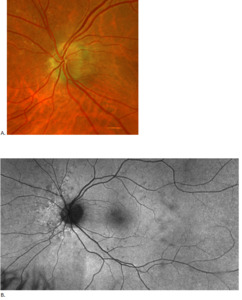
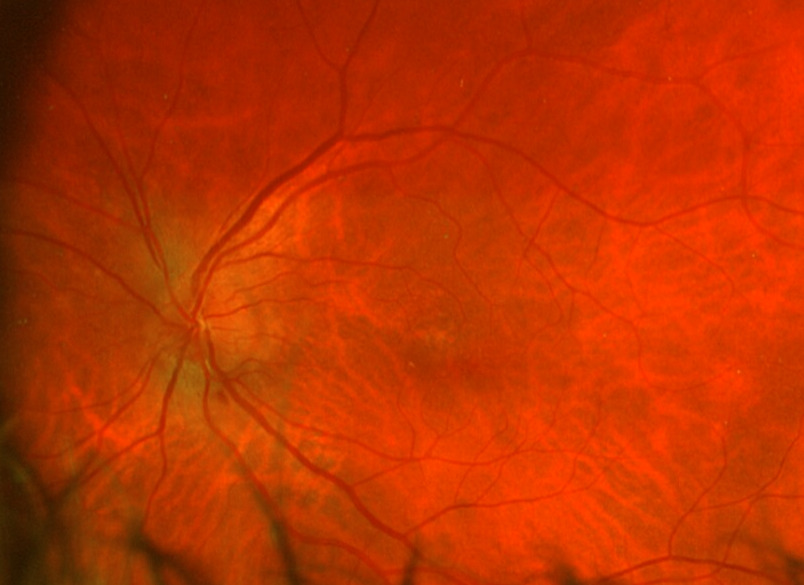
Posterior segment revealed IOL implants that were clear and centered after YAG Capsulotomy in both eyes. The optic nerve heads were both crowded with cupping of 0.10/0.10 (H/V) OU. The neuroretinal rim was intact in the right eye with no evidence of hemes, pallor, edema, or neovascularization of the disc. When observing the left eye, an inferior and inferior temporal rim hemorrhage was noted. Clinically, there was no apparent edema. There was a questionable area of swelling inferior temporal and superior temporal, but it was difficult to view. The vessels surrounding the optic nerve did not appear to be obscured and no spontaneous venous pulse was noted. The macular area was flat with even pigment and no clinically significant macular edema was present in either eye. Vessels displayed a normal AV ratio. The peripheral exam noted no holes, breaks, or tears in either eye. Finally, giant cell arteritis (GCA) symptoms of were ruled out.
At this visit a diagnosis of optic nerve head drusen (ONHD) or optic nerve head edema was considered. A follow-up was scheduled for a visual field, repeat ultrasound imaging, and other ancillary testing.
One Week Follow-Up
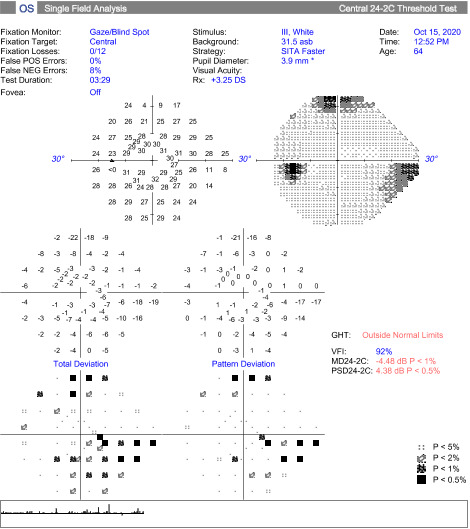
At the follow up, the patient’s vision remained stable without glasses, 20/20-1 OD and 20/25-1 OS. Humphrey Visual Field, Optos, Optos with autoflourescence, repeat B Scan, and color plates were performed at this visit. Color vision was normal in both eyes.
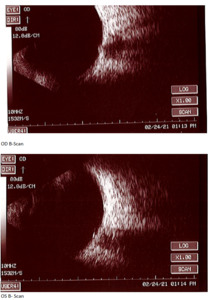
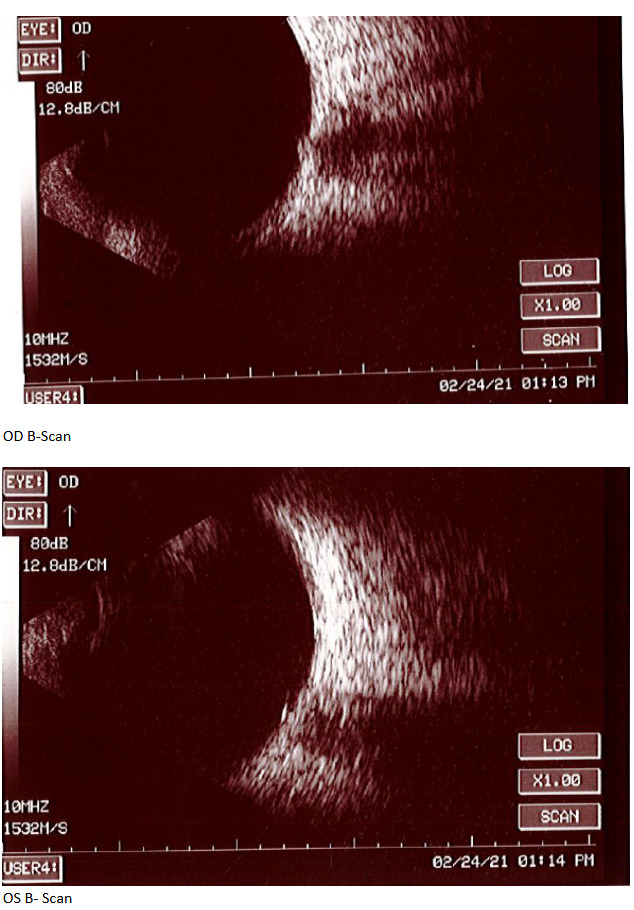
At the end of this visit, a diagnosis of optic nerve head drusen was determined due to the findings on the B-Scan. A highly reflective foci was seen due to the calcification present on the optic nerve head. There was no hyperflourescence seen on the fundus autoflourescent photos, but the drusen were likely buried. A 3-month follow-up was scheduled for repeat dilated fundus exam to ensure hemorrhages were resolving and no changes to the optic nerve head or visual acuity were occurring. The patient was educated on the condition of optic nerve head drusen and its effects on visual fields and vision. He was educated to return to clinic immediately if any changes were noted before the follow-up in 3 months.
Four Month Follow-Up
The patient presented for a dilated fundus exam and additional testing to view the resolving disc hemorrhage in the left eye. At this visit, he reported that he felt his eyes were worse and that they watered a lot. There was no change in his medical history.
His entering acuities without glasses was 20/40 OD, 20/40-2 OS. This was reduced from the previous examination. After refraction, best corrected visual acuity was 20/30 OD, 20/25- OS. This was still reduced in the right eye from the initial visit. All other testing was normal, including a careful pupil check including a .30 neutral density filter. This allowed us to look for even a mild afferent pupillary defect.
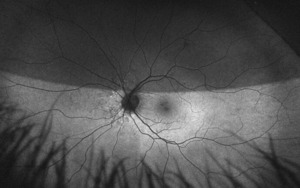
On this visit, the patient’s posterior pole revealed a blurred inferior temporal neuroretinal rim in both the left and right eyes. The right eye previously had no appearance of edema in the initial visit. The discs were crowded at .10/.10 OU as noted at initial visits and no hemorrhages were seen on this examination. All other structures posteriorly were consistent with the original examination.
Extensive testing was performed at this visit to ensure that disc edema was not a diagnosis. Unfortunately, the right eye photos are not available for this examination. OCT-A was also performed, but not included due to unreliability and poor quality of the scans.
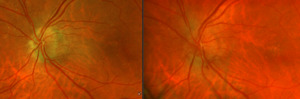
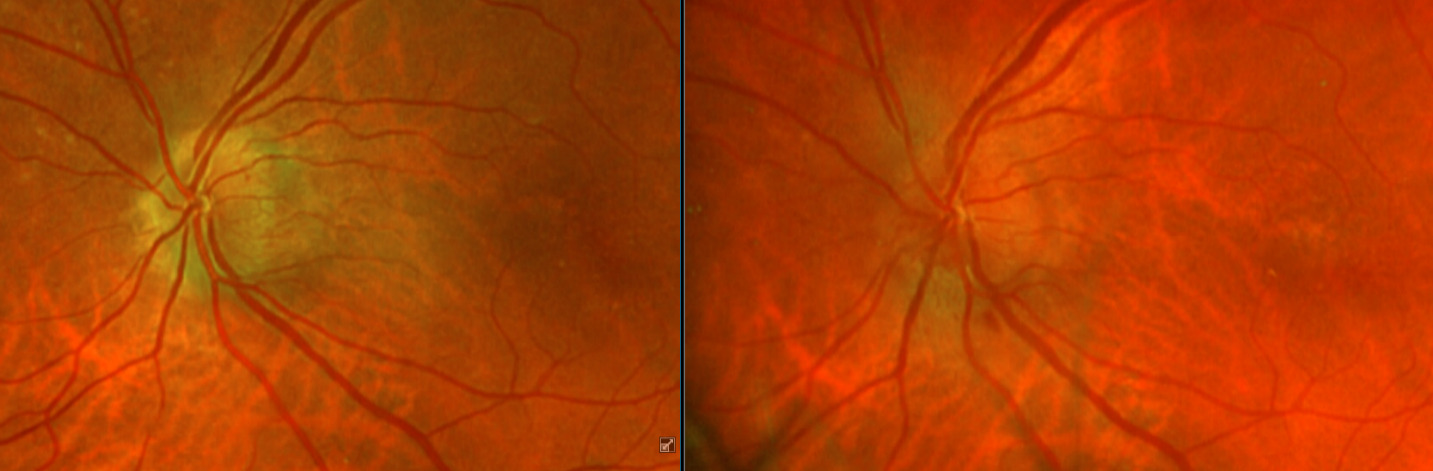
After additional testing was completed, and comparison of previous photos were conducted, it was determined that the patient had not only optic disc drusen, but also possible bilateral amiodarone optic neuropathy. The photos 4 months apart shows increasing pallor in the left eye and a blood vessel at 7 o’clock that did not look obscured initially but was evidently more pronounced on the follow up when comparing the initial and follow-up photos. This shows the importance of quality serial fundus photography.
Finally, when comparing the RNFL, it was noted that on the initial visit, there was RNFL thickening and on return, there was thinning and atrophy in both eyes. The return visit was further out than intended due to COVID-19. The thinning could be consistent with drusen, but more likely to be consistent with resolving NAION. Due to the insidious onset, lengthy recovery, and minimally reduced acuities, both diagnoses were considered.
A hand-off to the neuro-ophthalmologist noted similar findings. After discussing the case with him at length, it was confirmed that the patient not only had ONH drusen, but likely amiodarone related optic neuropathy as well. He advised contacting the patient’s primary care physician to discuss discontinuation, if possible, from Amiodarone.
After discontinuation of Amiodarone, the patient’s three-month follow-up showed improvement of vision to 20/25 in both eyes. The patient continues care at this time with neuro-ophthalmology.
Right image is original exam with inferior hemorrhage. Left image is 4-month follow-up visit. Resolution of the hemorrhage is seen, but atrophy of the disc overall should be noted. Vessel at 7 o’clock should also be noted. It appears much more pronounced than on initial visit.
Discussion
Optic Disc Drusen (ODD) are acellular, calcified deposits found in roughly 2.4% of the population. They are often bilateral in 75% of cases and there is no known gender predilection. The pathophysiology remains unknown and the condition is typically benign. Drusen is thought to be associated with retinitis pigmentosa, angiod streaks, and is present in up to 90% of patients with Alagille syndrome, but most patients do not have any predisposing conditions. ODD can display an autosomal dominant inheritance and they appear more commonly in the Caucasian population.1,2
Papilledema is classified as bilateral disc swelling secondary to raised intracranial pressure (ICP). Hemorrhages, cotton wool spots, and hyperemia of the optic nerve are frequently presenting factors. Spontaneous venous pulsation is usually absent. Initially, acuity is frequently preserved unless optic atrophy has developed, and a visual field will show an enlarged blind spot. A prompt workup must take place to ensure that grave pathologies are ruled out. Typically, a lumbar puncture must be performed to make an accurate diagnosis. Idiopathic intracranial hypertension is the most common cause of papilledema. Differentials include diabetic papillitis, non-arteritic ischemic optic neuropathy, and central retinal vein occlusion.
Roughly 60% of optics nerves with drusen have visible optic nerve head drusen (ONHD) on clinical examination.3 It is more common for the drusen to be located on the nasal side of the disc rather than the temporal sector. Drusen that are present beneath the disc’s surface can mimic a swollen disc and is the most common cause of pseudopapilledema. Differentiating between disc drusen and disc edema is of clinical importance due to the life-threatening nature of true disc edema versus the benign physiology of disc drusen. In most cases, a thorough examination and ancillary testing can rule out these disorders and avoid more invasive procedures such as lumbar puncture.
Typically, visual disturbances and headaches are uncommon with optic nerve head drusen. The optic nerve may appear elevated with indistinct margins, but the vessels along the nerve are not obscured. Headaches are very common with papilledema and can be associated with nausea and vomiting if the pressure is severe. The headache can worsen with change in position, such as reclining. Diplopia typically arises from a sixth nerve palsy.
Disc drusen are not present at birth but can appear early in childhood and late into adulthood. Drusen are typically buried in childhood and begin to become more exposed throughout the teenage years and into adulthood. It is not known if the drusen grow to become more exposed or if there is a loss of surrounding neural tissue that exposes the drusen with time. Crowded cupping and proximal branching as in the current case are common appearances of a nerve with disc drusen. Vessel tortuosity can also be seen. An APD could be present in asymmetric cases.4 The calcified lesions tend to grow slowly throughout life. Buried drusen that are located close to the lamina cribosa are difficult to diagnose on clinical examination and may require imaging to confirm the diagnosis. Because buried drusen are more prevalent in children and due to the increased difficulty in diagnosing this type of drusen, children are much more susceptible of misdiagnosis. Therefore, this puts children at a higher risk of enduring redundant testing for the evaluation of optic disc edema.5
Secondary complications can occur with disc drusen, but typically patients remain asymptomatic throughout life. Visual field defects are common in 24-87% of adults and up to 75% of cases and can progress with time, but patients do not normally notice the field loss.1 If a field defect is present, it is displayed as either an arcuate pattern, peripheral constriction, or an enlarged blind spot. The inferior nasal and inferior temporal quadrants are the most common arcuate defect location due to the compression of the optic nerve head fibers causing axonal loss. Visual field loss is most common in eyes with superficial drusen. As the disc drusen increase and become more exposed with time, there is a direct relationship with loss of field. Lee and Zimmerman reported a 1.6% per year increase of field loss throughout a 36-month period.6 Papilledema may produce and enlarged blind spot or visual blurring.
Rarely juxtapapillary choroidal neovascularization, vitreous hemorrhage, and vascular occlusions such as anterior ischemic optic neuropathy can occur. In up to 10% of cases with optic disc drusen, hemorrhages of the optic disc are seen.7 These hemorrhages include vitreal hemorrhages, splinter hemorrhages off the optic disc, and peripapillary hemorrhages that extend toward the macula. Papilledema typically causes flame hemorrhages, which are a fraction larger than splinter hemorrhages. It is still unknown why these hemorrhages with disc drusen occur, but it is speculated that drusen causes friction on the blood vessels and ultimately causes the vessels to leak or that the enlarging drusen causes venous stasis resulting in hemorrhage.
Multiple modes of imaging can be used to determine optic nerve head drusen. These include fundus autofluorescence (FAF), ultrasonography (US), optical coherence tomography (OCT), CT, fluorescein angiography (FA), and fundus photos.
FAF is impacted greatly on the volume and anatomical location of the disc drusen.8 FAF will show hyperfluorescent drusen if the drusen is close enough to the surface. Buried drusen do not auto fluoresce; therefore, FAF is not a reliable test to perform in children because of their higher incidence of being buried. This should not be the only form of testing to distinguish disc drusen as it has been stated that autofluorescence may only be present in as few as 12% of cases9
B-Scan will show the calcification as a highly reflective foci over the optic nerve using low gain. B-scan imaging has been found a superior test over FAF and computed tomography (CT) to differentiate ONHD and disc edema. It is easy to use, inexpensive, and can even be performed on children. Ultrasonography is dependent upon the calcium content of the drusen. Therefore, some studies have shown that less than 50% of cases of disc drusen are found by ultrasound.7 This is similar with CT scans as well. CT will amplify the drusen in its images if the calcification content is great enough, but is not a method of choice to solely determine optic disc drusen due to high cost, radiation exposure to the patient, and furthermore, any slices greater that 1.5 mm can easily miss disc drusen.7 Overall, B-scan is an excellent test to perform quickly in the determination of disc edema versus optic nerve head drusen, but in some cases drusen could be missed so this may not be the sole test in some cases. For years, B-scan has been considered the gold standard for the diagnosis of ODD, but with the addition of EDI-OCT, physicians may find themselves relying more heavily on newer technology. B-scans do not provide us with clear information about the structural integrity of the neural retina as the OCT does.
Distinguishing ODD from papilledema is extremely challenging, even for the most skilled eye-care professionals. The OCT gives clinicians the ability to better detect changes in drusen and the neural retina. This test allows scanning of the optic nerve head to the lamina cribosa and also decreases unnecessary and costly neurologic testing. In cases of disc edema and ONHD, the optic nerve head appears elevated. Disc edema optic nerve heads will result in a smooth ONH surface contour and ONHD will result in an irregular nerve contours. OCT generally shows a “lumpy bumpy” contour when relating to drusen versus papilledema. Papilledema OCT patterns are usually much smoother in nature. This however, is not a true verification tool. The Idiopathic Intracranial Hypertension Treatment Trial (IIHTT) found that 90% of eyes with papilledema have >/= 95 % thickening of RNFL. Thickening can occur with any form of acute disc edema. Conversely, OCT will display either a near normal, diffuse, or local thinning of the retinal nerve fiber layer, typically in the nasal sector with ODD.10 Eventually, RNFL thickness will decrease with disc edema either as a sign of improvement, or as a sign of axonal loss. ONHD can appear similar to blood vessels on the OCT. It is important to note that blood vessels are typically more oval shaped and also tend to produce a shadowing effect underneath.11
EDI-OCT allows deeper images in the retina and optic nerve to be observed with greater detail than with the conventional OCT. This image allows the clinician to find buried drusen with better resolution and can allow for the drusen’s shape and structure to be assessed. This is thought that it can eventually help with visual function, as opposed to B-scan, which only shows structure and anatomy. EDI-OCT has shown to be a more reliable diagnostic modality in ONHD diagnosis than B-scan which was previously the gold standard.9
The Optic Disc Drusen Studies (ODDS) Consortium was established in 2015 and was developed to make recommendations regarding the diagnosis of ODD and the EDI-OCT. The following interpretations from the EDI-OCT were taken from the ODDS research. ODDS Consortium shows that the optic disc drusen are always located at the level above the lamina cribosa and that drusen have a signal poor core. Disc drusen are often seen with a hyperreflective margin, most prominently in the superior aspect. Drusen are occasionally interpreted as conglomerates of smaller optic disc drusen with internal reflectivity within the poor signaling core. Hyperreflective horizontal lines might represent early disc drusen but should not be diagnosed as so. Peripapillary hyperreflective ovoid mass-like structures (PHOMS) should not be diagnosed as optic disc drusen. The ODDS Consortium also recommend that clinicians use 6-line radial scans to observe the RNFL thickness.12
OCT-A is currently being studied as well. It has been shown that the superficial capillary network associated with the optic disc can reveal focal attenuation with the more superficial ODD. It is unknown, but hypothesized, that the attenuation of the vessels could correspond to compression or displacement of the capillary plexus by the optic disc drusen.13
Fluorescein angiography is much less used with advancements in technology to determine disc drusen due to its invasiveness. Although, it still remains useful if there is ever a doubt that disc edema is present. The drusen will autofluorescence in the pre-injection phase and after the dye is injected, the margin of the disc will be well-defined, except where the overlying drusen are located. Buried drusen will not autofluorescence. There will be an irregular peripapillary hyperfluorescence during the late phase with drusen. Disc edema will present with a diffuse, early fluorescein leakage.1 The dye will remain around the nerve and will not seep into the surrounding retina with disc drusen as it does with papilledema.
There is no treatment for disc drusen, but luckily, patients do not often suffer from visual field loss. Clinical observation is needed to ensure that secondary complications are diagnosed quickly and managed timely. If managed timely, patients with papilledema will typically have an improvement, or even resolution, of their visual field.
Optic disc drusen presence can predispose patients to low tension glaucoma and vascular complications such as AION, CRAO and CRVO. Optic disc drusen can present many complications regarding glaucoma. With crowded anatomical features at the disc, enlarged cupping can be hidden. Also, OCT RNFL displays thinning with both drusen and glaucoma. With visual field defects, it is difficult to determine if the defect is secondary to the mechanical stress of drusen or glaucomatous involvement. If any defect is present and glaucoma is suspected, the recommendation is to treat for glaucoma by lowering the intraocular pressures. Brimonidine has demonstrated neuroprotective properties in relation to the retinal ganglion cells in glaucoma.9
Crowding of the optic nerve head may lead to impaired blood flow and can predispose a patient to vascular events such as AION, CRAO and CRVO.14 These conditions typically affect adults later in age, but in the case of ODD, much younger populations, including children, can be affected. Vasoactive therapy is currently being studied for these conditions and their relation to ODD. If a choroidal neovascular membrane is found secondary to chronic ischemia, anti-VEGF treatment is utilized. All the above vascular events can lead to severe visual field loss.
Although optic nerve head drusen was the suspected primary diagnosis in both eyes initially in this case, it is worth discussing the involvement of Amiodarone and its ocular drug-induced side effects. Amiodarone is a cardiac antiarrhythmic drug. Amiodarone is most notably seen in the anterior segment as golden-brown deposits in the anterior cornea in a whorl-like pattern. This is called verticillate keratopathy, or “whorl keratopathy.” It is present in roughly 70-100% of patients taking the drug.15 Visual complaints due to these deposits are uncommon, but occasionally the symptomatic patient will have complaints of photophobia, hazy vision, colored halos around lights, or glare. Macular edema can be seen as well, although not common. If these occur and patients seem to have an increased sensitivity, discontinuation of the drug can occur.
In this case, side effects were seen in the context of optic neuropathy. Amiodarone associated optic neuropathy occurs in approximately 1-2% of patients taking the drug.16 It is unknown if Amiodarone is a variant of NAION or if it is an independent risk factor. NAION is typically a unilateral, acute and sudden vision loss with resolution of disc edema within a few weeks. Amiodarone induced optic neuropathy is described as an insidious onset with slower regression. The disc edema is prolonged with a median duration of 3 months and typically the occurrence is bilateral.16 Hemorrhages seen with amiodarone optic neuropathy also tend to persist for several months whereas NAION associated hemorrhages resolve in a much shorter amount of time. There is typically no sex predilection with NAION, but amiodarone-associated neuropathy has shown to be more prevalent in men. Finally, vision loss is quite variable, ranging from mild and possibly reversible loss to severe and permanent loss.17
In a review of amiodarone related optic neuropathy, Passman et al found that patients typically can take the drug for 9 months before visual symptoms occur. Also, 1/3 of patients were asymptomatic and 44% of patients had an insidious onset of optic neuropathy. Disc edema was present in 85% of cases and after the drug was stopped, 58% had improved visual acuity, 21% remained the same acuity, and 21% progressed. The half-life for this drug is up to 100 days and it has been noted that optic nerve head edema can persist for several months following discontinuation of the drug.17,18
According to Purvin et al 2006, there are several groups of patients in the management of patients taking amiodarone. Group 1 patients display bilateral disc edema and if all testing is negative to rule out increased intracranial pressure and giant cell arteritis, it should be strongly encouraged to discontinue amiodarone. In group 2, unilateral NAION is present with an insidious onset. If the cup-to-disc in the fellow eye is not crowded, the disc edema is lengthy, and mild optic nerve dysfunction is present, then discontinuation should be strongly considered. Finally, in group 3 patients, unilateral NAION with immediate onset of disc edema, the presence of a crowded contralateral eye with no symptoms of systemic involvement from amiodarone, and moderate to severe optic nerve involvement can consider continuation of the drug. Other sources state that if any patient has optic neuropathy that was induced by amiodarone, the discontinuation of the drug is necessary and another form of treatment for the patient’s cardiac symptoms should be used. There is currently no treatment for optic neuropathy.14
Conclusion
Occasionally, one finds that a single diagnosis may not be the only ailment present. A patient can have multiple disease processes and it takes clinical judgement and a thorough understanding of ancillary testing to determine the appropriate diagnosis, or diagnoses, as in this case. Optic disc drusen is a common finding in the routine eye exam, but it can greatly mimic much more grave diagnoses. As a wise professor once said, “A patient can have as many diseases as he pleases.” Interdisciplinary collaboration and additional testing and follow ups were key in making the diagnosis for this patient.